Figure 3.
The TMV MP C-Terminal Domain Contains Major Phosphorylation Sites Recognized by PAPK.
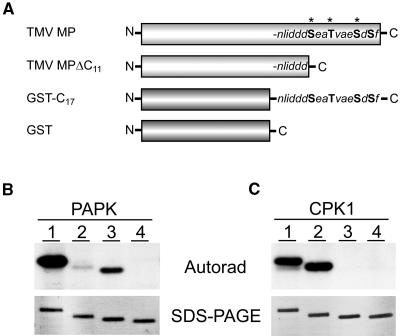
(A) Schematic diagram illustrating the recombinant proteins used to identify the phosphorylation sites, present in TMV MP, recognized by PAPK. TMV MP: general structure indicating the positions of Ser/Thr residues in the C terminus (bold), including those previously identified as being in vivo phosphorylation sites (asterisks; Ser258, Thr261, and Ser265; Waigmann et al., 2000). TMV MPΔC11: deletion mutant lacking the 11 C-terminal residues. GST-C17:GST fusion protein containing the 17 C-terminal residues of the TMV MP. GST: control protein for our phosphorylation reactions.
(B) PAPK recognized, specifically, the Ser/Thr residues within the C terminus of TMV MP. Lane 1, TMV MP (30 kD); lane 2, TMV MPΔC11 (29 kD); lane 3, GST-C17 (28 kD); lane 4, GST (26 kD). Each phosphorylation assay contained 5 μL of heparin column-purified PAPK fraction and 1 μg of substrate protein in a 50-μL reaction volume.
(C) TMV MP C terminus lacked phosphorylation substrate sites for pumpkin CPK1. Parallel assays to those in (B) performed using 20 ng of purified recombinant pumpkin CPK1. Substrate phosphorylation visualized by autoradiography (top panels) and purified substrate proteins used in these assays (1 μg each) are shown in GelCode Blue–stained SDS-PAGE gels (bottom panels).