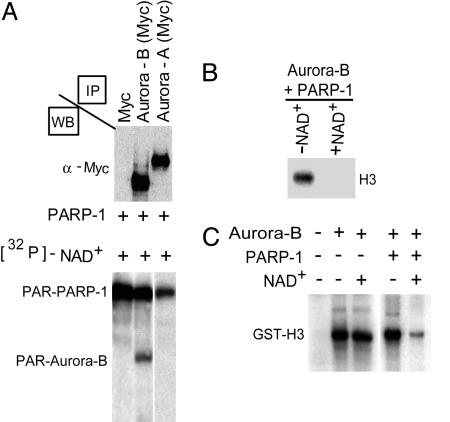
Fig. 4.
Poly(ADP-ribosyl)ation of Aurora-B blocks kinase activity. (A) Myc-tagged Aurora-A or Aurora-B kinases immunoprecipitated from COS-1 transfected cells were used as substrates in a poly(ADP-ribosyl)ation reaction catalyzed by PARP-1 using [32P]-NAD. Aurora-B is readily poly(ADP-ribosyl)ated by PARP-1, whereas Aurora-A is not. The reaction products were separated on SDS-polyacrylamide gel and visualized by autoradiography. Western blot analyses (WB) with an anti-Myc antibody revealed comparable amounts of expressed recombinant Aurora-A and Aurora-B. PAR, poly(ADP-ribosyl)ated. (B) Aurora-B immunoprecipitated from COS-1 cells was subjected to poly(ADP-ribosyl)ation in the presence or absence of NAD+, and its kinase activity was evaluated by using [32P]-γATP and a mix of histones as a substrate. Levels of phosphorylated H3 were detected by autoradiography. Only in the presence of NAD+ is the histone kinase activity of the poly(ADP-ribosyl)ated Aurora-B drastically inhibited, as shown by the absence of H3 phosphorylation. (C) GST-Aurora-B was similarly used for kinase assays using GST-H3 as a substrate in the presence or absence of NAD+ and/or PARP-1. Phosphorylation of GST-H3 was revealed by autoradiography. No difference was observed when the activity of Aurora-B was measured in the presence or absence of NAD+ alone. Kinase activity decreased after poly(ADP-ribosyl)ation by PARP-1.