Abstract
In mice and humans, a normal offspring can be obtained by injecting a single spermatozoon into an oocyte, the process called intracytoplasmic sperm injection (ICSI). When three or more mouse spermatozoa with intact acrosomes were injected into individual mouse oocytes, an increasing proportion of oocytes became deformed and lysed. Oocytes did not deform and lyse when acrosome-less spermatozoa were injected, regardless of the number of spermatozoa injected. Injection of more than four human spermatozoa into a mouse oocyte produced vacuole-like structures in each oocyte. This vacuolation did not happen when spermatozoa were freed from acrosomes before injection. Hamsters, cattle, and pigs have much larger acrosomes than the mouse or human. Injection of a single acrosome-intact hamster, bovine, and porcine spermatozoon deformed and lysed many or all mouse oocytes. This deformation did not happen when these spermatozoa were freed from acrosomes before ICSI, regardless of the number of spermatozoa injected. Because trypsin and hyaluronidase mimicked the action of acrosome-intact spermatozoa, it is likely that the acrosomal enzymes deform and lyse the oocytes. Injection of small amounts of trypsin and hyaluronidase into normally fertilized mouse eggs disturbed their pre- and postimplantation development. In view of potentially harmful effects of acrosomal enzymes on embryo development, the removal of acrosomes before ICSI is recommended for animals with large sperm acrosomes. The removal of acrosomes may increase the efficiency of ICSI in these animals. Although human and mouse spermatozoa do not need to be freed from acrosomes, the removal of acrosomes before ICSI is theoretically preferable.
Keywords: assisted fertilization, bovine, human, mouse, porcine
Intracytoplasmic sperm injection (ICSI) has become the method of choice to treat human male infertility when all other forms of assisted fertilization have failed (1). Many childless couples who are unable to conceive by conventional in vitro fertilization and other treatments now have a fairly good chance to have their own children by ICSI. Animals in which ICSI has been successfully used to produce live offspring include mice (2), golden hamsters (3), rats (4), rabbits (5), cattle (6), sheep (7), horses (8), cats (9), pigs (10), and monkeys (11).
The acrosome is a cap-like structure covering the anterior portion of the sperm head (12). It contains an array of hydrolyzing enzymes (12, 13). Immediately before normal fertilization, both the sperm plasma membrane covering the acrosome and the contents of the acrosome are shed as a result of the acrosome reaction (12). Thus, the acrosome and its contents never enter the oocyte under normal conditions. Therefore, it is rather surprising that ICSI can produce apparently normal offspring because it usually introduces the entire acrosome into the ooplasm. In the hamster, prior removal of the acrosome from the spermatozoon is essential for successful ICSI. Injection of an acrosome-intact spermatozoon results in the death of the oocyte (3). Although injection of acrosome-intact spermatozoa of other species (e.g., mouse and human) does not kill the oocytes, we cannot rule out the possibility that the contents of the acrosome may have some effects on the development of embryos.
In this study, we investigated how mouse oocytes respond to injection of a single or multiple spermatozoa with or without their acrosomes. The effects of the injection of hydrolyzing enzymes (trypsin and hyaluronidase) on oocytes and embryos were also examined.
Materials and Methods
Reagents and Media. All inorganic and organic reagents were purchased from Sigma unless otherwise stated. The medium used for culturing oocytes after ICSI was bicarbonate-buffered Chatot, Ziomet and Bavister (CZB) medium supplemented with 5.56 mM d-glucose and 4 mg/ml BSA (14). The medium used for oocyte collection and ICSI was modified CZB with 20 mM Hepes-Na, 5 mM NaHCO3, and 0.1 mg/ml poly(vinyl alcohol) (PVA; cold water-soluble) instead of BSA. CZB was used under 5% CO2 in air, and Hepes-CZB under 100% air. The pH value of these media was ≈7.4. The reason for using CZB medium instead of the currently popular potassium simplex optimized medium (KSOM) medium is that the 10 mM SrCl2 we used to activate mouse oocytes tends to precipitate in KSOM medium. As long as we use hybrid mice, we do no see any differences between these two media in their ability to support in vitro development of fertilized eggs.
Animals. Mice and Syrian (golden) hamsters were maintained in accordance with the guidelines of the Laboratory Animal Service at the University of Hawaii and those prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resource National Research Council [Department of Health, Education, and Welfare publication (National Institutes of Health) 80-23, revised 1985]. The protocol describing our animal handling and treatment procedures was reviewed and approved by the Animal Care and Use Committee at the University of Hawaii.
Preparation of Mouse Oocytes. Female B6D2F1 mice, 8–12 weeks of age, were induced to superovulate by i.p. injection of 7.5 units of equine chorionic gonadotropin (eCG) followed 48 h later by i.p. injection of 7.5 units of human chorionic gonadotropin (hCG). Mature oocytes were collected from the oviducts 14–15 h after hCG injection. They were freed from the cumulus cells by 3-min treatment with 0.1% (wt/vol) bovine testicular hyaluronidase (300 USP units/mg; ICN) in Hepes-CZB. The cumulus-free oocytes were thoroughly rinsed and kept in CZB medium before ICSI for up to 3 h at 37°C. Only the oocytes with disintegrated first polar bodies were used for ICSI to make later identification of activated and unactivated oocytes easier by the presence or absence of the second polar body.
Preparation of Spermatozoa, ICSI, and Examination of ICSI Oocytes. A dense sperm mass from the cauda epididymides of the mouse or hamster was placed at the bottom of a 1.5-ml centrifuge tube containing 300 μl of CZB medium. The spermatozoa were allowed to swim up into the medium for 3–15 min at 37°C. The supernatant containing actively motile spermatozoa was then transferred to 500-μl microtubes. A straw (0.5 ml) of frozen bull spermatozoa was thawed in a 37°C water bath for 1 min. Thawed bull semen was placed at the bottom of a 1.5-ml centrifuge tube containing 500 μl of CZB to allow the spermatozoa to swim into the medium for 20 min at 37°C. Boar spermatozoa were isolated from ejaculated semen of fertile males. A 500-μl aliquot of the semen was placed at the bottom of a 1.5-ml centrifuge tube containing 500 μlofCZBto allow spermatozoa to swim up for 20 min at 37°C. For human spermatozoa, 1.5 ml of liquid human semen was placed at the bottom of a 5-ml centrifuge tube containing 3 ml of CZB. The spermatozoa were allowed to swim up for 60 min at 37°C. ICSI was carried out according to Kimura and Yanagimachi (2) with some modifications. A small amount (25 μl) of the swim-up sperm suspension was mixed thoroughly with 50 μl of Hepes-CZB containing 8–12% (wt/vol) poly(vinyl pyrrolidone) (PVP) (Mr 360,000). A drop of this suspension was transferred under mineral oil (Squibb) in a plastic dish (100 mm in diameter and 10 mm in depth) previously placed on the stage of an inverted microscope equipped with a micromanipulator. A single spermatozoon of the mouse or hamster was drawn tail first into an injection pipette, and the head was separated from the tail by applying a few piezo pulses to the neck region. The isolated sperm head was injected immediately into an oocyte. Only the sperm head is needed to obtain offspring by ICSI (15). A single human, bull, or boar spermatozoon, drawn into the pipette, was immobilized by applying a few piezo pulses to its midpiece region before the entire body of the spermatozoon was injected into a mouse oocyte. When more than two spermatozoa were injected into a single oocyte, care was taken to minimize the volume of the medium injected. Approximately 30 oocytes in a group were operated on within 15 min. ICSI was completed within 2 h aftercollection of the oocytes from oviducts. In some experiments, acrosomes were removed before ICSI by vortexing mouse, bull, boar, or human spermatozoa for 1 min in Hepes-CZB containing 1% Triton X-100 (16). Some porcine spermatozoa were sonicated for 1 min (four 15-s bursts at 10-s intervals) by using 50% power output at 0°C in Hepes-CZB containing 2 mg/ml lysolecithin. After washing with Triton-free Hepes-CZB by centrifugation (2,000 × g, 3 min), two or more acrosome-less sperm heads were injected into each oocyte. ICSI oocytes were examined 5–7 h later with an interference-contrast microscope for the presence or absence of pronuclei and the second polar body. The oocytes with second polar bodies were recorded activated. Some oocytes were compressed between a slide and coverslip, fixed briefly with 2% glutaraldehyde, stained with acetocarmine, and mounted in acetic glycerol to reconfirm the number and state of pronuclei (17). Some other oocytes were cultured in vitro for 3 or more days to see whether they continue to survive. The LIVE/DEAD Cell Viability Test Kit (L-7013, Molecular Probes) was used to distinguish live and dead oocytes/embryos.
Examination of the Effects of Enzymes Injected into Oocytes. We chose trypsin and hyaluronidase as enzymes to be tested because trypsin is similar to acrosin in its substrate cleavage specificity and hyaluronidase is commonly extracted from mammalian testes (spermatozoa). Trypsin (trypsin 2.5% liquid, Invitrogen) and hyaluronidase (300 USP units/mg; ICN) were separately injected into mouse oocytes. The amounts of trypsin and hyaluronidase injected into each oocyte were 1–40 pg and 80–500 pg, respectively. BSA was also injected into mouse oocytes as a control. The amount of BSA injected into each oocyte was ≈550 pg. Oocytes were examined 5–7 h after injection as described.
Immunolocalization of Microtubules and Microfilaments in Oocytes After ICSI and Enzyme Injection. Immunostaining of microtubules and microfilaments was performed as described (18–21) with some modifications. In brief, the oocytes were fixed at room temperature for 40 min with 2% (wt/vol) formaldehyde in Dulbecco's PBS containing both 0.1% PVA and 0.2% Triton X-100. After washing three times in PBS with PVA (PBS-PVA), they were stored in PBS-PVA containing 1% BSA (PBS-PVA-BSA) for up to 4 days at 4°C. For immunodetection of microtubules and microfilaments, the oocytes were incubated for 30 min at room temperature with monoclonal anti-α-tubulin antibody produced in mouse (T-9026, Sigma), which was diluted 1:500 with PBS-PVA-BSA. After three washings with PBS-PVA-BSA containing 0.5% Triton X-100, oocytes were incubated with Alexa Fluor 488-labeled goat anti-mouse IgG (A-11029, Molecular Probes), at a dilution of 1:200, in PBS-PVA-BSA for 20 min at room temperature or Cy-5-labeled goat anti-mouse antibody (Jackson ImmunoResearch) at a dilution of 1:200 in PBS-PVA-BSA for 20 min at room temperature. After three washings with PBS-PVA-BSA containing 0.5% Triton X-100, actin distribution was analyzed by using phalloidin-fluorescein isothiocyanate (P-5282, Sigma). Oocytes were incubated for 60 min at room temperature with antibody diluted 1:250 in PBS. After three washings with PBS-PVA-BSA, oocyte nuclei were labeled with 10 mg/ml propidium iodide (PI) (P-4170, Sigma) in PBS for 90 min at room temperature. After three washings with PBS-PVA-BSA, they were mounted between a slide and coverslip. Alexa Fluor 488 and PI emitted green and red fluorescence, respectively. Cy5 originally generated a red fluorescence signal (670 nm), but this red fluorescence was converted to blue in images. This conversion allowed us to distinguish between Cy5 and PI. Preparations were viewed by using a Bio-Rad MRC-1024 confocal scanning laser microscope. At least 25 oocytes from more than four animals were used for each experiment.
In Vitro Fertilization (IVF). Cumulus-enclosed oocytes, collected from oviducts ≈15 h after human chorionic gonadotropin injection, were inseminated in Toyoda, Yokoyama and Hoshi (TYH) medium (22). Sperm concentration at insemination was 2–4 × 106/ml. Oocytes were examined for evidence of fertilization 2 h later as already described for ICSI oocytes. The conditions we used resulted in normal fertilization in 73.3% (151 of 206) of the oocytes. In a separate series of experiments, 1.4 pg of trypsin or 100 pg of hyaluronidase was injected into the cytoplasm of a fertilized egg at the early pronuclear stage (≈6 h after IVF).
Embryo Transfer After ICSI. Two-cell embryos developed from normally fertilized oocytes were transferred into oviducts of pseudopregnant CD1 (albino) females that had been mated during the previous night with vasectomized males of the same strain. After embryo transfer, the surrogate females were killed on day 19 of pregnancy, and their uteri were examined for the presence of live fetuses. Live fetuses, if any, were collected by Caesarian section and raised by lactating CD1 foster mothers.
Statistical Analysis. The difference between the experimental group and matched control group was compared by using Fisher's exact probability test. Differences were considered significant at the P < 0.05 level.
Results
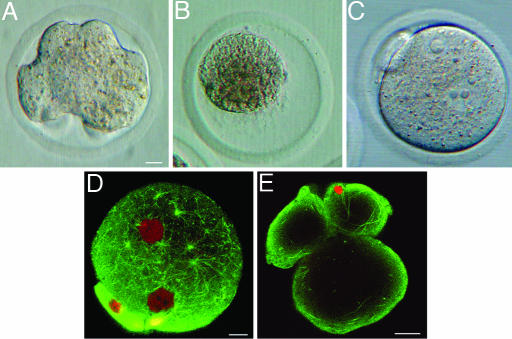
Response of Mouse Oocytes to the Injection of a Single or Multiple Mouse Spermatozoa. Ordinarily for mouse ICSI, a single spermatozoon (2) or single isolated sperm head (15) is injected into each oocyte. In this study, the majority of mouse oocytes injected with a single sperm head were activated normally and developed into blastocysts (Table 1, Exp. A). When 2 acrosome-intact sperm heads were injected into each oocyte, the proportion of the oocytes reaching the blastocyst stage was drastically reduced (Table 1, Exp. B). When three or more acrosome-intact sperm heads were injected, increasing proportions of the oocytes became deformed (Table 1, Exps. C–E, and Fig. 1A) starting from several hours after ICSI. Although deformed oocytes were alive (determined by the cell viability test), a few or none reached the blastocyst stage. When 8 or more acrosome-intact sperm heads were injected into each oocyte, all oocytes became deformed and remained in the same state for 3 days. Injection of 10 or more acrosome-intact sperm heads resulted in oocyte deformation, edema, and eventual cytolysis of all oocytes (Fig. 1B). None of the oocytes became deformed when acrosome-less sperm heads were injected, irrespective of the number of the heads injected (Table 1, Exps. F–I, and Fig. 1C). Deformation of the ooplasm after injection of multiple sperm heads was not due to the introduction of an excess volume of the medium injected because the introduction of a large volume of the medium itself did not deform oocytes (Table 1, Exps. K and L). In normally fertilized oocytes, many astral microtubules were distributed throughout the ooplasm (Fig. 1D). In the oocytes that became deformed after injection of several acrosome-intact sperm heads, fibrous microtubules were lying near the oocyte cortex (Fig. 1E). Distribution of actins in the oocyte was the same in both single-sperm-injected and multiple-sperm-injected oocytes (data not shown).
Table 1. Response of mouse oocytes to the injection of a single and multiple mouse spermatozoa.
| No. of eggs developed to
|
||||||
|---|---|---|---|---|---|---|
| Exp. | No. of sperm heads injected into a single oocyte | Total no. of oocytes injected (no. of reps.) | No. of oocytes survived ICSI and activated (%) | No. of oocytes deformed extensively 7 h after ICSI (%) | 2-cell (%) | Blastocyst (%) |
| Acrosome-intact | ||||||
| A | 1 | 197 (9) | 168 (85) | 0 (0) | 160 (95)a | 136 (81)b |
| B | 2 | 94 (11) | 83 (88) | 0 (0) | 71 (86)c | 41 (49)d |
| C | 3 | 59 (10) | 49 (83) | 16 (33) | 19 (39)e | 1 (2)f |
| D | 4–7 | 90 (12) | 66 (73) | 37 (56) | 0 (0) | 0 (0) |
| E | 8–12 | 21 (7) | 14 (67) | 14 (100) | 0 (0) | 0 (0) |
| Acrosome-less | ||||||
| F | 1 | 49 (9) | 39 (80) | 0 (0) | 35 (90) | 24 (62) |
| G | 2 | 42 (8) | 29 (69) | 0 (0) | 27 (93) | 19 (66) |
| H | 3 | 18 (8) | 10 (56) | 0 (0) | 6 (60) | 3 (30) |
| I | 4–7 | 37 (9) | 25 (68) | 0 (0) | 10 (40) | 2 (8) |
| J | >8* | – | – | – | – | – |
| K | 0† | 57 (7) | –‡ | 0 (0) | 0 (0) | 0 (0) |
| L | 1§ | 90 (7) | 75 (83) | 0 (0) | 61 (81) | 46 (61) |
Superscript letters: P < 0.01 for a vs. c, a vs. e, and b vs. d; P < 0.001 for b vs. f.
Acrosome-less sperm heads were so “sticky” to each other that injection of more than seven sperm heads was technically impossible
Sham operation: 2 × 10–5 μl of Hepes CZB containing PVP (100 mg/ml) was injected into each oocyte. This volume of the medium corresponded roughly to that of the medium injected with 2–12 sperm heads
Fifty-two (91%) oocytes survived, but all of the surviving oocytes did not activate
A single sperm head in 2 × 10–5 μl of Hepes CZB containing PVP (100 mg/ml) was injected into each oocyte
Fig. 1.
Mouse oocytes each injected with a single or multiple mouse sperm heads. (A) A deformed oocyte 7 h after injection of four acrosome-intact sperm heads. (B) An oocyte cytolyzed after injection of 10 acrosome-intact sperm heads. (C) An oocyte injected with 4 acrosome-less sperm heads. This oocyte did not deform and contained a few large pronuclei. (D) Confocal image of an oocyte 7 h after normal IVF. Note many asterous tubules in the ooplasm. Pronuclei are shown as large red spherical structures. (E) Confocal image of an oocyte injected with six acrosome-intact sperm heads. Note that microtubules are lying near the cortex of the deformed oocyte and are absent in the interior part of the oocyte. Pronuclei are not in this optical section. [Scale bars: 10 μm (A–C), 10 μm (D), and 10 μm (E).]
Response of Mouse Oocytes to the Injection of Hamster, Bovine, and Porcine Spermatozoa With or Without Acrosomes. The observation that the injection of a single acrosome-intact hamster sperm head causes a drastic deformation of a mouse oocyte (16) was confirmed by the present study (Table 2, Exp. A, and Fig. 2 A and B). In deformed oocytes, microtubules were heavily concentrated in the cortex of the oocyte. Oocytes did not deform when an acrosome-less hamster sperm head was injected (Fig. 2C and Table 2, Exp. C). Similar results were obtained after injection of bovine and porcine spermatozoa. Injection of a single acrosome-intact spermatozoon deformed nearly half or more of the oocytes (Table 2, Exps. D and J). Three or more acrosome-intact spermatozoa cytolyzed one-third or all of the oocytes (Table 2, Exps. F and K), whereas acrosome-less spermatozoa did not (Table 2, Exp. I and L).
Table 2. Response of mouse oocytes to the injection of a single and multiple hamster, bovine, and porcine spermatozoa.
| No. of oocytes deformed extensively (%)
|
No. of oocytes cytolyzed (%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Exp. | No. of sperm injected into each oocyte | Status of acrosome | Total no. of oocytes injected (no. of reps.) | No. of oocytes survived ICSI and activated (%) | 7 h after ICSI | 24 h after ICSI | 7 h after ICSI | 24 h after ICSI | No. of eggs developed to 2-cell (%) |
| Hamster | A | 1 | Acrosome-intact | 47 (6) | 30 (64) | 30 (100) | 30 (100) | 0 (0) | 0 (0) | 0 (0) |
| B | 3 | Acrosome-intact | 10 (3) | 4 (40) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 0 (0) | |
| C | 1 | Acrosome-less | 50 (5) | 38 (76) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 38 (100) | |
| Bovine | D | 1 | Acrosome-intact | 100 (5) | 65 (65) | 18 (28) | 30 (46) | 0 (0) | 1 (2)a | 2 (3) |
| E | 2 | Acrosome-intact | 31 (4) | 15 (48) | 5 (33) | 11 (73) | 1 (7) | 1 (7) | 0 (0) | |
| F | 3–7 | Acrosome-intact | 17 (4) | 9 (53) | 9 (100) | 9 (100) | 3 (33) | 4 (44)b | 0 (0) | |
| G | 1 | Acrosome-less | 51 (4) | 17 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (12) | |
| H | 2 | Acrosome-less | 21 (5) | 11 (52) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | |
| I | 3–7* | Acrosome-less | 7 (3) | 3 (43) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Porcine | J | 1 | Acrosome-intact | 109 (4) | 50 (46) | 44 (88) | 44 (88)c | 1 (2) | 33 (66) | 2 (4)d |
| K | 3 | Acrosome-intact | 18 (3) | 15 (83) | 15 (100) | 15 (100) | 15 (100) | 15 (100) | 0 (0) | |
| L | 1 | Acrosome-less† | 79 (4) | 35 (44) | 0 (0) | 4 (11)e | 0 (0) | 0 (0) | 27 (77)f | |
Superscript letters: P < 0.001 for c vs. e, a vs. b, and d vs. f.
Injection of more than three acrosome-less spermatozoa was difficult due to adhesion of spermatozoa
Triton X-100 (1%) was used to remove acrosomes of hamster and bovine spermatozoa. This was not strong enough to remove acrosomes from all porcine spermatozoa, and therefore we used lysolecithin instead (see Materials and Methods). It was possible that, even with lysolecithin, acrosomes were not removed completely in some spermatozoa as suggested by deformation of some oocytes 24 h after ICSI
Fig. 2.
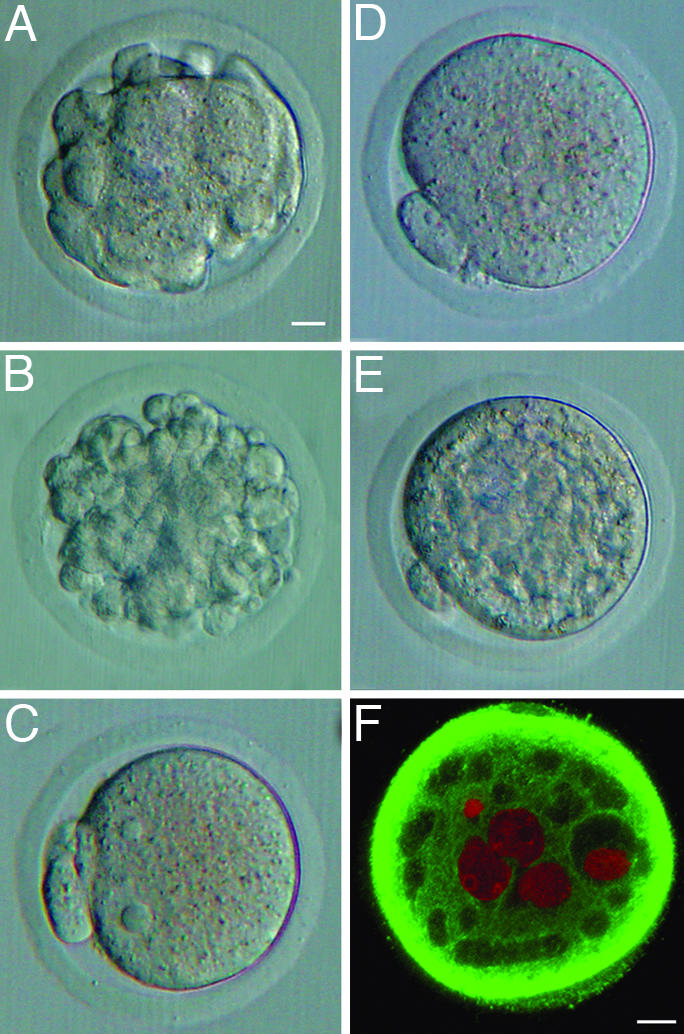
Mouse oocytes injected with hamster or human spermatozoa. (A)An oocyte injected with a single acrosome-intact hamster sperm head, 4 h after ICSI, with extensive deformation. (B) An oocyte injected with a single acrosome-intact hamster sperm head, 24 h after ICSI, with extensive deformation. (C) An oocyte injected with a single acrosome-less hamster sperm head, 4 h after ICSI, without deformation. (D) An oocyte injected with a single acrosome-intact human spermatozoon, 6 h after ICSI, without deformation. (E)An oocyte injected with six acrosome-intact human spermatozoa, 20 h after ICSI, with irregular appearance of the cytoplasm. (F) Confocal section of an oocyte injected with six acrosome-intact human spermatozoa, 20 h after ICSI, showing many “vacuoles” within the cytoplasm. Sperm pronuclei are shown as red spherical structures. (A–E) Interference-contrast microscopy. (F) Confocal scanning laser microscopy. [Scale bars: 10 μm (A–E) and 10 μm (F).]
Response of Mouse Oocytes to the Injection of Human Spermatozoa. Unlike other spermatozoa, a single human spermatozoon injected into an oocyte did not deform the oocyte (Figs. 2D). However, injection of four or more human spermatozoa produced “vacuole-like” structures within the ooplasm (Table 3, Exps. B–D, and Fig. 2 E and F). No vacuoles were formed when acrosome-less spermatozoa were injected (Table 3, Exp. E). None of the mouse oocytes injected with a single or multiple human spermatozoa developed beyond the two-cell stage.
Table 3. Response of mouse oocytes to the injection of a single or multiple human spermatozoa.
| No. of eggs developed (%)
|
|||||||
|---|---|---|---|---|---|---|---|
| Exp. | No. of human sperm injected into a single mouse oocyte | Status of acrosome | Total no. of oocytes injected (no. of reps.) | No. of oocytes survived ICSI and activated (%) | No. of oocytes with many vacuole-like structures 30 h after ICSI (%) | To 2-cell | Beyond 2-cell |
| A | 1–3 | Acrosome-intact | 103 (6) | 62 (60) | 0 (0) | 51 (82)a | 0 (0) |
| B | 4 | Acrosome-intact | 35 (5) | 17 (49) | 4 (24)b | 10 (59) | 0 (0) |
| C | 5–6 | Acrosome-intact | 25 (6) | 16 (64) | 11 (69)c | 5 (31)d | 0 (0) |
| D | 7–8 | Acrosome-intact | 11 (6) | 7 (64) | 7 (100) | 0 (0) | 0 (0) |
| E | 7–8* | Acrosome-less | 10 (2) | 5 (50) | 0 (0) | 0 (0) | 0 (0) |
| F | 0† | – | 40 (4) | –‡ | 0 (0) | 0 (0) | 0 (0) |
| G | 1§ | Acrosome-intact | 50 (4) | 34 (68) | 0 (0) | 27 (79) | 0 (0) |
Superscript letters: P < 0.05 for b vs. c; P < 0.001 for a vs. d.
Seven to eight acrosome-less sperm were injected into each oocyte
Sham operation. A total of 2 × 10–5 μl of HEPES-CZB containing PVP (100 mg/ml) was injected into each oocyte. This volume of the medium corresponded roughly to that of the medium injected with two to eight spermatozoa
Forty (100%) oocytes survived, but all surviving oocytes did not activate
A single spermatozoon in 2 × 10–5 μl of HEPES-CZB containing PVP (100 mg/ml) was injected into each oocyte
Response of Mouse Oocytes to the Injection of Enzymes. Reactions of mouse oocytes to the injection of various enzymes are summarized in Table 4. The oocytes each injected with >1.4 pg of trypsin deformed within 5 min and then cytolyzed (Table 4, Exp. B). Microtubules were accumulated extensively in the oocyte cortex of these deformed oocytes. Heated trypsin did not deform the oocytes (Table 4, Exp. C). Oocytes injected with >250 pg of hyaluronidase activated within 2 h and then deformed within 5 h (Table 4, Exp. E). Injection of BSA (>550 pg) had no effects (Table 4, Exp. F).
Table 4. Response of mouse oocytes to the injection of trypsin or hyaluronidase.
| State of oocytes, examined 5–7 h after injection
|
|||||
|---|---|---|---|---|---|
| Amounts injected into each oocyte | Exp. | Total no. of oocytes injected (no. of reps.) | No. survived (%) | No. activated* (%) | No. deformed (%) |
| Trypsin | |||||
| 1.4 pg | A | 25 (3) | 24 (96) | 0 (0) | 0 (0) |
| 1.4–40 pg | B | 81 (6) | 0 (0) | 0 (0) | 75 (100) |
| 30 pg (heated)† | C | 23 (2) | 20 (87) | 0 (0) | 0 (0) |
| Hyaluronidase | |||||
| 80–250 pg | D | 34 (3) | 33 (97) | 0 (0) | 0 (0) |
| 250–500 pg | E | 55 (3) | 50 (90) | 50 (100) | 40 (80) |
| BSA | |||||
| ≈550 pg | F | 25 (2) | 20 (80) | 0 (0) | 0 (0) |
Oocytes with one pronucleus and the second polar body
Heated at 100°C for 2 min
Development of IVF-Fertilized Eggs Injected with Trypsin or Hyaluronidase. Sperm acrosome extract (1.2–3.6 pg) and Hepes-CZB medium injected into the cytoplasm of normally fertilized eggs did not disturb pre- and postimplantation development of embryos (Table 5, Exps. A and D), but trypsin (1.4 pg) and hyaluronidase (100 pg) significantly affected both pre- and postimplantation development of embryos (Table 5, Exps. B and C).
Table 5. Effects of trypsin, hyaluronidase, and sperm acrosome extract (SPA) injected into IVF-fertilized pronuclear eggs on embryo development.
| No. of zygotes developed to
|
||||||
|---|---|---|---|---|---|---|
| Exp. | Material injected into each zygote | Total no. of zygotes cultured (no. of reps.) | 2-cell (%) | Blastocyst (%) | No. of 2-cell transferred (no. of recipients) | No. of live offspring (%) |
| A | Mouse SPA (1.2–3.6 pg) | 30 (2) | 29 (97) | 29 (97) | 58 (5) | 40 (69) |
| B | Trypsin (1.4 pg) | 87 (5) | 39 (44) | 23 (26)a | 72 (5) | 24 (33)b |
| C | Hyaluronidase (100 pg) | 55 (2) | 31 (56) | 21 (38)c | 36 (2) | 6 (17)d |
| D | HEPES-CZB* | 20 (2) | 20 (100) | 18 (90) | 26 (2) | 20 (77) |
| E | None (control) | 143 (6) | 140 (98) | 126 (88)e | 27 (2) | 21 (78)f |
Superscript letters: P < 0.001 for a vs. e, c vs. e, b vs. f, and d vs. f.
Sham operation: 2 × 10–5 μl of HEPES-CZB was injected into each IVF-fertilized zygote
Discussion
In the human and mouse, injection of a single spermatozoon into an oocyte can result in the birth of a healthy offspring (1, 2, 23). This is not the case for the Syrian hamster. Injection of a single acrosome-intact spermatozoon into each oocyte inevitably causes lysis of all oocytes within 2 h (3). Removal of acrosomes is a key to success of hamster ICSI. Although ICSI has been successful for many other species (24), no systematic study has been conducted to determine whether the presence or absence of the acrosomes in spermatozoa affects the outcome of ICSI.
Here, we report that mouse oocytes became deformed and cytolyzed when three or more acrosome-intact mouse spermatozoa were injected into each oocyte. However, they did not deform when injected spermatozoa (sperm heads) were free of acrosomes (Table 1). Abnormal accumulation of microtubules in the cortex of oocyte (Fig. 1E) could be a cause of the deformation and subsequent cytolysis of oocytes. Because acrosome-intact bovine and porcine spermatozoa cytolyzed mouse oocytes, whereas acrosome-less spermatozoa did not (Table 2), it must be the acrosome, not spermatozoa per se, that caused the problem. The minimum number of acrosome-intact spermatozoa (or sperm heads) that deformed 100% of mouse oocytes by ICSI were as follows: one (hamster), two (porcine), three (bovine), seven (human), and eight (mouse). This order (hamster, porcine, bovine, human, and mouse) seems to be correlated to the volume of the acrosome, with that in hamster spermatozoa being the largest. The components of the acrosome harmful to the oocyte are likely to be acrosomal enzymes such as trypsin-like acrosin and hyaluronidase because injection of commercially available trypsin and hyaluronidase mimicked the effects of supernumerary acrosome-intact spermatozoa (Table 4). We preliminarily tested mouse sperm acrosome extracts prepared according to Baba et al. (25) and found that they induce oocyte activation without deforming the oocytes (data not shown). We suspect that the concentration of acrosin in the sperm extracts was too low to deform the oocytes. Oocyte activation could be due to either acrosomal hyaluronidase or contamination of calcium ionophore used for the induction of the sperm acrosome reaction. Molecular mechanisms by which acrosomal materials and exogenous enzymes damage the oocyte's cytoskeletal system and disturb embryo development must be the subject of future investigation.
Because a single spermatozoon is injected during human and mouse ICSI, its acrosomal enzymes released into the oocyte would not impose a serious problem. Fortune has smiled on human and mouse ICSI because the acrosomes of these species happen to be rather small (24). If human sperm acrosomes were as large as hamster or bull sperm acrosomes and if human oocytes (≈7 × 105 μm3) were as small as mouse oocytes (≈2.7 × 105 μm3), human ICSI could have been problematic from its inception. Even though removal of the acrosome from an individual spermatozoon before ICSI is not essential in the human or the mouse, it is theoretically preferable because the acrosome and its contents never enter the oocyte under natural conditions. It is expected that spermatozoa of infertile men have either normal or below normal levels of acrosomal enzymes such as acrosin (26–28). However, spermatozoa of some infertile men may have excess amounts of acrosomal enzymes. It is possible that oocytes of some infertile women have cytoplasm extremely vulnerable to the damage caused by exogenous hydrolyzing enzymes. In such cases, ICSI may result in abnormal development or even death of embryos. Such abnormal development could be avoided by selecting a few normal-looking motile spermatozoa, removing their acrosomes by a brief treatment with a membrane-disrupting agent (such as nonionic detergent or lysolecithin), followed by thorough rinsing and immediate ICSI. The report that embryo development and pregnancy are better after the injection of acrosome-reacted human spermatozoa than acrosome-intact spermatozoa† is consistent with the notion described in this paper and deserves reconfirmation.
The efficiency of ICSI (the proportion of live offspring developed from all ICSI oocytes) in species other than humans and mice is rather low: ≈15% at best (24). The removal of acrosomes before ICSI may increase its efficiency. It is very important to keep sperm nuclei undamaged during or after the removal of the acrosomes. Excess exposure of demembranated (acrosome-less) spermatozoa to harsh reagents or prolonged maintenance of demembranated spermatozoa in an inappropriate medium may damage sperm nuclei, thus resulting in the failure, rather than the improvement, of ICSI.
Acknowledgments
We thank Professor Eimei Sato, Dr. Hiromichi Matsumoto, and Ms. Yumi Hoshino of Tohoku University for teaching K.M. the methods of immunostaining cytoskeltons and providing us with valuable information. We thank Professor Tadashi Baba and Dr. Ekyune Kim for the preparation of mouse sperm extracts. We thank Dr. Chin N. Lee and Dr. Halina Zaleski of the University of Hawaii College of Agriculture for supplying bovine and porcine spermatozoa and Dr. Steven Ward and Mrs. Renata Prisztoka of the Institute for Biogenesis Research for supplying hamster spermatozoa. We thank Mrs. Tina Carvalho for her valuable advice on confocal microscopy. We thank Dr. Stanley Meizel (University of California, Davis) and Dr. George Gerton (University of Pennsylvania) for information about acrosomal enzymes. We are indebted to Dr. John R. McCarrey (University of Texas, San Antonio), Dr. George Seidel (Colorado State University), and Dr. Vincent De Feo and Mrs. Charlotte Oser (University of Hawaii) for reading the original manuscript. We also thank Dr. Stefan Moisyadi and Mr. Boris Umylny for their assistance in the preparation of the original manuscript. This study was supported by Grants from the Harold Castle Foundation and University of Hawaii Research Training and Revolving Funds (RTRF).
Author contributions: K.M. and R.Y. designed research; K.M. performed research; K.M. and R.Y. analyzed data; and K.M. and R.Y. wrote the paper.
Abbreviations: ICSI, intracytoplasmic sperm injection; CZB, Chatot, Ziomet, and Bavister medium; PVA, poly(vinyl alcohol); IVF, in vitro fertilization; PVP, poly(vinyl pyrrolidone).
Footnotes
Lee, D. R., Lim, Y. L., Lee, J. E., Kim, H. J., Joon, H. S. & Roh, S. I., 52nd Annual Meeting of the American Society of Reproductive Medicine, Nov. 2–6, 1996, Boston, abstr. p-008.
References
- 1.Van Steirteghem, A. C., Nagy, Z., Joris, H., Liu, J., Staessen, C., Smitz, J., Wisanto, A. & Devroey, P. (1993) Hum. Reprod. 8, 1061-1066. [DOI] [PubMed] [Google Scholar]
- 2.Kimura, Y. & Yanagimachi, R. (1995) Biol. Reprod. 52, 709-720. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi, Y., Yanagimachi, R. & Horiuchi, T. (2002) Biol. Reprod. 67, 534-539. [DOI] [PubMed] [Google Scholar]
- 4.Said, S., Han, M. S. & Niwa, K. (2003) Theriogenology 60, 359-369. [DOI] [PubMed] [Google Scholar]
- 5.Deng, M. & Yang, X. J. (2001) Mol. Reprod. Dev. 59, 38-43. [DOI] [PubMed] [Google Scholar]
- 6.Goto, K., Kinoshita, A., Takuma, Y. & Ogawa, K. (1990) Vet. Rec. 127, 517-520. [PubMed] [Google Scholar]
- 7.Catt, S. L., Catt, J. W., Gomez, M. C., Maxwell, W. M. & Evans, G. (1996) Vet. Rec. 139, 494-495. [DOI] [PubMed] [Google Scholar]
- 8.Li, X., Morris, L. H. & Allen, W. R. (2001) Reproduction 121, 925-932. [PubMed] [Google Scholar]
- 9.Pope, C. E., Johnson, C. A., McRae, M. A., Keller, G. L. & Dresser, B. L. (1998) Anim. Reprod. Sci. 53, 221-236. [DOI] [PubMed] [Google Scholar]
- 10.Martin, M. J. (2000) Biol. Reprod. 63, 109-112. [DOI] [PubMed] [Google Scholar]
- 11.Hewitson, L., Dominko, T., Takahashi, D., Martinovich, C., Ramalho-Santos, J., Sutovsky, P., Fanton, J., Jacob, D., Monteith, D., Neuringer, M., et al. (1999) Nat. Med. 5, 431-433. [DOI] [PubMed] [Google Scholar]
- 12.Yanagimachi, R. (1994) The Physiology of Reproduction (Raven, New York), 2nd Ed., pp. 189-317.
- 13.Zaneveld, L. & De Jonge, C. (1991) in A Comparative Overview of Mammalian Fertilization, eds. Dunbar, B. S. & O'Rand, M. G. (Plenum, New York), pp. 63-79.
- 14.Chatot, C. L., Ziomek, C. A., Bavister, B. D., Lewis, J. L. & Torres, I. (1989) J. Reprod. Fertil. 86, 679-688. [DOI] [PubMed] [Google Scholar]
- 15.Kuretake, S., Kimura, Y., Hoshi, K. & Yanagimachi, R. (1996) Biol. Reprod. 55, 789-795. [DOI] [PubMed] [Google Scholar]
- 16.Kimura, Y., Yanagimachi, R., Kuretake, S., Bortkiewicz, H., Perry, A. C. & Yanagimachi, H. (1998) Biol. Reprod. 58, 1407-1415. [DOI] [PubMed] [Google Scholar]
- 17.Yanagida, K., Yanagimachi, R., Perreault, S. D. & Kleinfeld, R. G. (1991) Biol. Reprod. 44, 440-447. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto, H., Jiang, J. Y., Mitani, D. & Sato, E. (2002) J. Exp. Zool. 293, 641-648. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino, Y., Yokoo, M., Yoshida, N., Sasada, H., Matsumoto, H. & Sato, E. (2004) Mol. Reprod. Dev. 69, 77-86. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto, H., Shoji, N., Umezu, M. & Sato, E. (1998) J. Exp. Zool. 281, 149-153. [DOI] [PubMed] [Google Scholar]
- 21.Shinozawa, T., Mizutani, E., Tomioka, I., Kawahara, M., Sasada, H., Matsumoto, H. & Sato, E. (2004) Mol. Reprod. Dev. 68, 313-318. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda, Y., Yokoyama, M. & Hoshi, T. (1971) Jpn. J. Anim. Reprod. 16, 147-151. [Google Scholar]
- 23.Palermo, G., Joris, H., Devroey, P. & Van Steirteghem, A. C. (1992) Lancet 340, 17-18. [DOI] [PubMed] [Google Scholar]
- 24.Yanagimachi, R. (2005) Reprod. Biomed. Online 10, 247-288. [DOI] [PubMed] [Google Scholar]
- 25.Baba, D., Kashiwabara, S., Honda, A., Yamagata, K., Wu, Q., Ikawa, M., Okabe, M. & Baba, T. (2002) J. Biol. Chem. 277, 30310-30314. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal, A. & Loughlin, K. R. (1991) Arch. Androl. 27, 97-101. [DOI] [PubMed] [Google Scholar]
- 27.Bartoov, B., Reichart, M., Eltes, F., Lederman, H. & Kedem, P. (1994) Andrologia 26, 9-15. [DOI] [PubMed] [Google Scholar]
- 28.Langlois, M. R., Oorlynck, L., Vandekerckhove, F., Criel, A., Bernard, D. & Blaton, V. (2005) Clin. Chim. Acta 351, 121-129. [DOI] [PubMed] [Google Scholar]