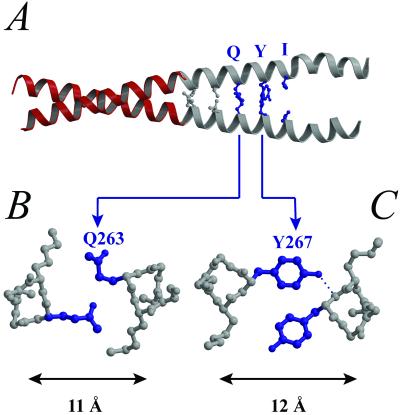
Figure 2.
Structural basis for the splaying in the C-terminal region of striated muscle Tm. (A) In the C-terminal region of striated muscle α-Tm, there are three consecutive “core” residues (blue) that are generally disfavored in two-stranded coiled coils: Gln-263 (d position), Tyr-267 (a position), and Ile-270 (d position). The two equivalent Ile side chains do not contact each other, and the pairs of Gln and Tyr side chains do not display the usual symmetric “knobs-into-holes” packing pattern. In each of these two cases (B and C), only one of the two equivalent residues is inserted into the hole on the opposite helix. Moreover, the distance between the two helices increases to accommodate the insertion of such long or bulky side chains.