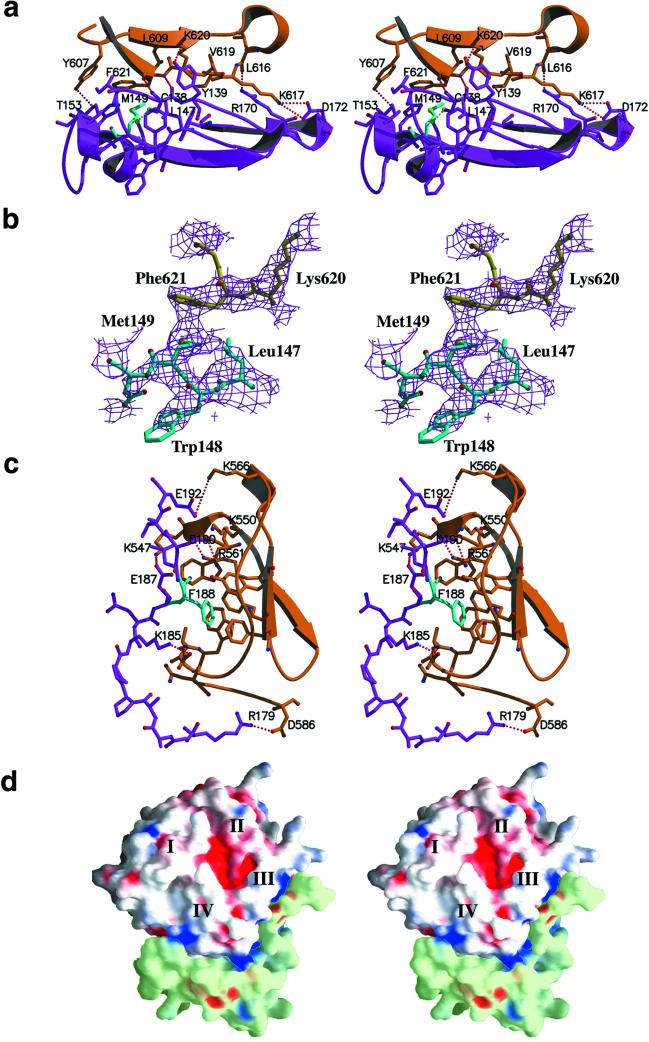
Figure 2.
Contact areas in the proMMP-2/TIMP-2 complex. To clarify the details of binding areas, the orientation of the complex in a–c was turned on 180o compared with the so-called standard orientation presented in Fig. 1. (a) Stereo view of the GH loop of TIMP-2 (magenta) in contact with the fourth blade of the hemopexin β-propeller of proMMP-2 (orange). Met-149 of TIMP-2, a central residue in the hydrophobic interaction, colored cyan, forms a close contact with Phe-621 of proMMP-2. Red dashed lines illustrate hydrogen bonds and salt bridges, and the green dashed line represents an S-S bridge formed by Cys-133–Cys-138. (b) Stereo view of the 2 Fo − Fc electron density map (contoured at 1.0 σ) with the model structure shows the hydrophobic contact between Met-149 (blue) molecules and Phe-621 (yellow). (c) The C terminus of TIMP-2 (magenta) is inserted between the third and fourth blades of the hemopexin domain (orange). Phe-188 (cyan) is buried in a hydrophobic groove formed by aromatic side chains on the surface of the hemopexin domain. Red dashed lines illustrate hydrogen bonds and salt bridges. (d) Stereo view of the grasp (37) representation of the contact areas between hemopexin domain of proMMP-2 (white) and C-terminal domain of TIMP-2 (light green). Molecular surfaces corresponding to positive potential are colored blue and areas with negative potential are shown in red. The negatively charged C tail of TIMP-2 is positioned in a positively charged cluster formed by residues of blade III of the hemopexin domain.