Abstract
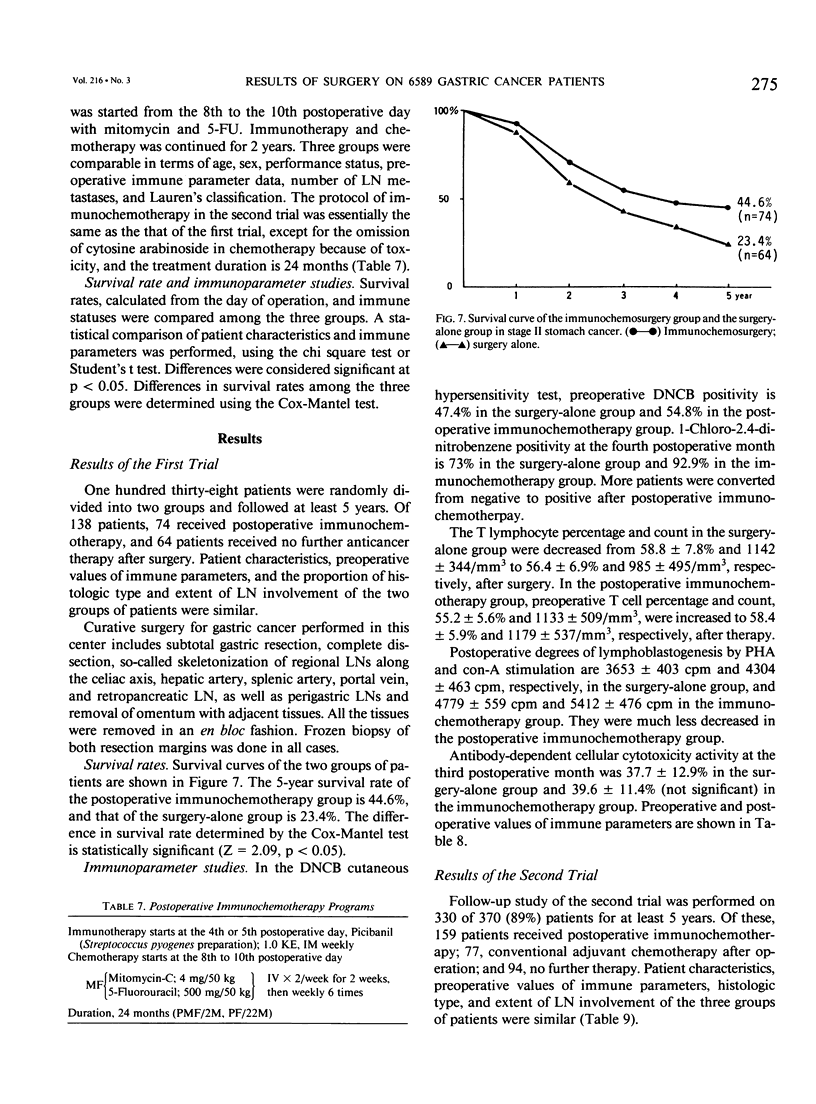
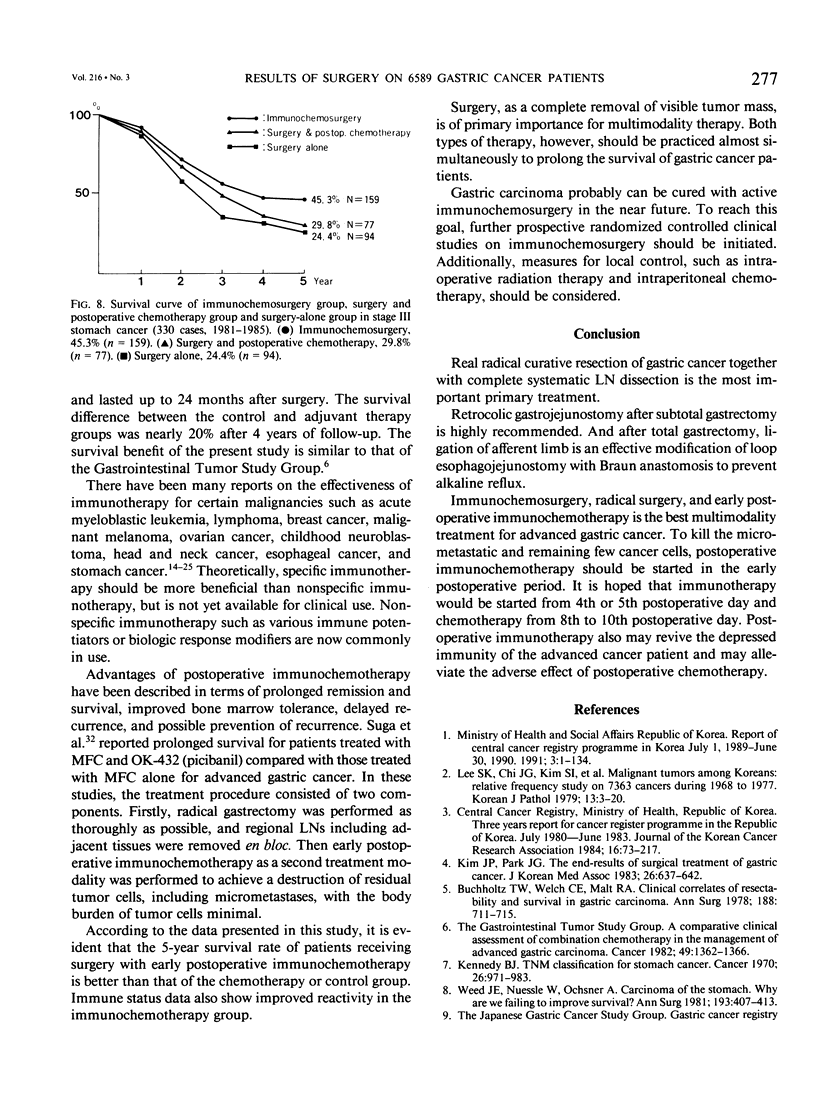
Results of 6589 gastric cancer operations at the Department of Surgery, Seoul National University Hospital, from 1970 to 1990 were reported. About two thirds (76.6%) were advanced gastric cancer (stages III and IV). The 5-year survival rate of operated stage III gastric cancer was only 30.6%, with frequent recurrence. Conversely, cell-mediated immunities of advanced gastric cancer patients were significantly decreased. Therefore, to improve the cure rate and to prevent or delay recurrence, curative surgery with confirmation of free resection margins and systematic lymph node dissection of perigastric vessels were performed and followed by early postoperative immunotherapy and chemotherapy (immunochemosurgery) in stage III patients. To evaluate the effect of immunochemosurgery, two randomized trials were studied in 1976 and 1981. In first trial, 5-fluorouracil, mitomycin C, and cytosine arabinoside for chemotherapy and OK 432 for immunotherapy were used. The 5-year survival rates for surgery alone (n = 64) and immunochemosurgery (n = 73) were 23.4% and 44.6%, respectively, a significant difference. In the second trial, there were three groups: group I, immunochemosurgery (n = 159); group II, surgery and chemotherapy (n = 77); and group III, surgery alone (n = 94). 5-Fluorouracil and mitomycin C for chemotherapy and OK-432 for immunotherapy were administered for 2 years. The 5-year survival rate of group I was 45.3%, significantly higher than the 29.8% of group II and than the 24.4% of group III. The postoperative 1-chloro-2.4-dinitrobenzene test, T-lymphocyte percentage, phytohemagglutinin- and con-A-stimulated lymphoblastogenesis and the antibody-dependent cell-mediated cytotoxicity test showed more favorable values in the immunochemosurgery group. Therefore, immunochemosurgery is the best multimodality treatment for advanced gastric cancer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchholtz T. W., Welch C. E., Malt R. A. Clinical correlates of resectability and survival in gastric carcinoma. Ann Surg. 1978 Dec;188(6):711–715. doi: 10.1097/00000658-197812000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman J. U., Cardenas J. O., Blumenschein G. R., Hortobagyi G., Burgess M. A., Livingston R. B., Mavligit G. M., Freireich E. J., Gottlieb J. A., Hersh E. M. Chemoimmunotherapy of advanced breast cancer: prolongation of remission and survival with BCG. Br Med J. 1976 Nov 20;2(6046):1222–1225. doi: 10.1136/bmj.2.6046.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman J. U., Mavligit G. M., Blumenshein G., Burgess M. A., McBride C. M., Hersh E. M. Immunotherapy of human solid tumors with Bacillus Calmette-Guérin: prolongation of disease-free interval and survival in malignant melanoma, breast, and colorectal cancer. Ann N Y Acad Sci. 1976;277(00):135–159. doi: 10.1111/j.1749-6632.1976.tb41695.x. [DOI] [PubMed] [Google Scholar]
- Hattori T., Mori A., Hirata K., Ito I. Five-year survival rate of gastric cancer patients treated by gastrectomy, large dose of mitomycin-C, and-or allogeneic bone marrow transplantation. Gan. 1972 Oct;63(5):517–522. [PubMed] [Google Scholar]
- Kennedy B. J. T N M classification for stomach cancer. Cancer. 1970 Nov;26(5):971–983. doi: 10.1002/1097-0142(197011)26:5<971::aid-cncr2820260503>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- MacDonald J. S., Woolley P. V., Smythe T., Ueno W., Hoth D., Schein P. S. 5-fluorouracil, adriamycin, and mitomycin-C (FAM) combination chemotherapy in the treatment of advanced gastric cancer. Cancer. 1979 Jul;44(1):42–47. doi: 10.1002/1097-0142(197907)44:1<42::aid-cncr2820440108>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Mathé G. Active immunotherapy. Adv Cancer Res. 1971;14:1–36. doi: 10.1016/s0065-230x(08)60517-5. [DOI] [PubMed] [Google Scholar]
- Moertel C. G., Mittelman J. A., Bakemeier R. F., Engstrom P., Hanley J. Sequential and combination chemotherapy of advanced gastric cancer. Cancer. 1976 Aug;38(2):678–682. doi: 10.1002/1097-0142(197608)38:2<678::aid-cncr2820380209>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Eilber F. R., Holmes E. C., Sparks F. C., Ramming K. BCG immunotherapy as a systemic adjunct to surgery in malignant melanoma. Med Clin North Am. 1976 May;60(3):431–439. doi: 10.1016/s0025-7125(16)31890-9. [DOI] [PubMed] [Google Scholar]
- Morton D., Eilber F. R., Malmgren R. A., Wood W. C. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery. 1970 Jul;68(1):158–164. [PubMed] [Google Scholar]
- Nissen-Meyer R., Kjellgren K., Malmio K., Månsson B., Norin T. Surgical adjuvant chemotherapy: results with one short course with cyclophosphamide after mastectomy for breast cancer. Cancer. 1978 Jun;41(6):2088–2098. doi: 10.1002/1097-0142(197806)41:6<2088::aid-cncr2820410604>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Okudaira Y., Sugimachi K., Inokuchi K., Kai H., Kuwano H., Matsuura H. Postoperative long-term immunochemotherapy for esophageal carcinoma. 5 year survival. Jpn J Surg. 1982;12(4):249–256. doi: 10.1007/BF02469556. [DOI] [PubMed] [Google Scholar]
- Orita K., Miwa H., Fukuda H., Yumura M., Uchida Y. Preoperative cell-mediated immune status of gastric cancer patients. Cancer. 1976 Dec;38(6):2343–2348. doi: 10.1002/1097-0142(197612)38:6<2343::aid-cncr2820380621>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Powles R. L., Crowther D., Bateman C. J., Beard M. E., McElwain T. J., Russell J., Lister T. A., Whitehouse J. M., Wrigley P. F., Pike M. Immunotherapy for acute myelogenous leukaemia. Br J Cancer. 1973 Nov;28(5):365–376. doi: 10.1038/bjc.1973.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman S. P., Livingston R. B., Gutterman J. U., Suen J. Y., Hersh E. M. Chemotherapy versus chemoimmunotherapy of head and neck cancer: report of a randomized study. Cancer Treat Rep. 1976 May;60(5):535–539. [PubMed] [Google Scholar]
- Rosenberg S. Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J Natl Cancer Inst. 1985 Oct;75(4):595–603. [PubMed] [Google Scholar]
- Suga S., Tsunekawa H., Washino M., Makino N., Tamura Z., Goto S. Treatment of gastric cancer, with special reference to the survivals of the cancer patients treated with multiple combination MFC therapy or immunochemotherapy of MFC plus OK-432 (NSC B116209). Gastroenterol Jpn. 1977;12(2):20–29. doi: 10.1007/BF02773621. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Thaler H. T., Hansen J. A., Rosen P. P., Robbins G. F., Urban J. A., Oettgen H. F., Good R. A. Immunologic reactivity in patients with primary operable breast cancer. Cancer. 1978 Jan;41(1):84–94. doi: 10.1002/1097-0142(197801)41:1<84::aid-cncr2820410113>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Weed T. E., Nuessle W., Ochsner A. Carcinoma of the stomach. Why are we falling to improve survival? Ann Surg. 1981 Apr;193(4):407–413. doi: 10.1097/00000658-198104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]


