Abstract
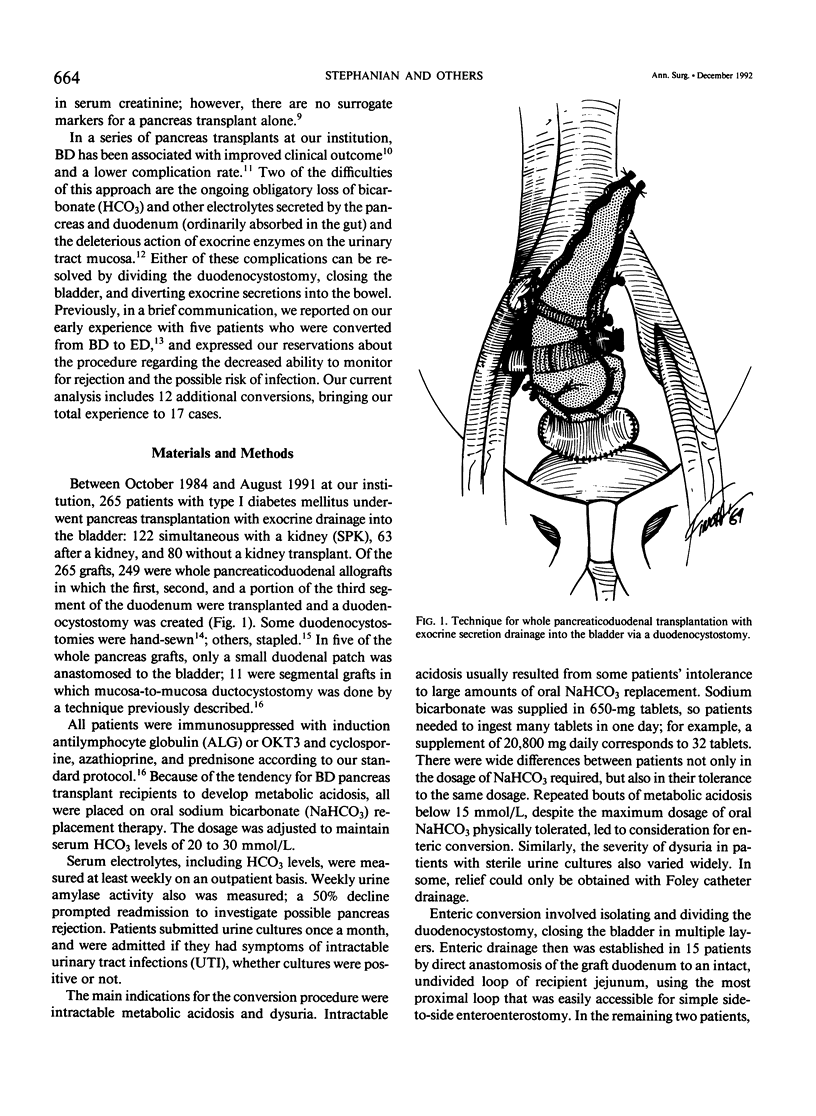
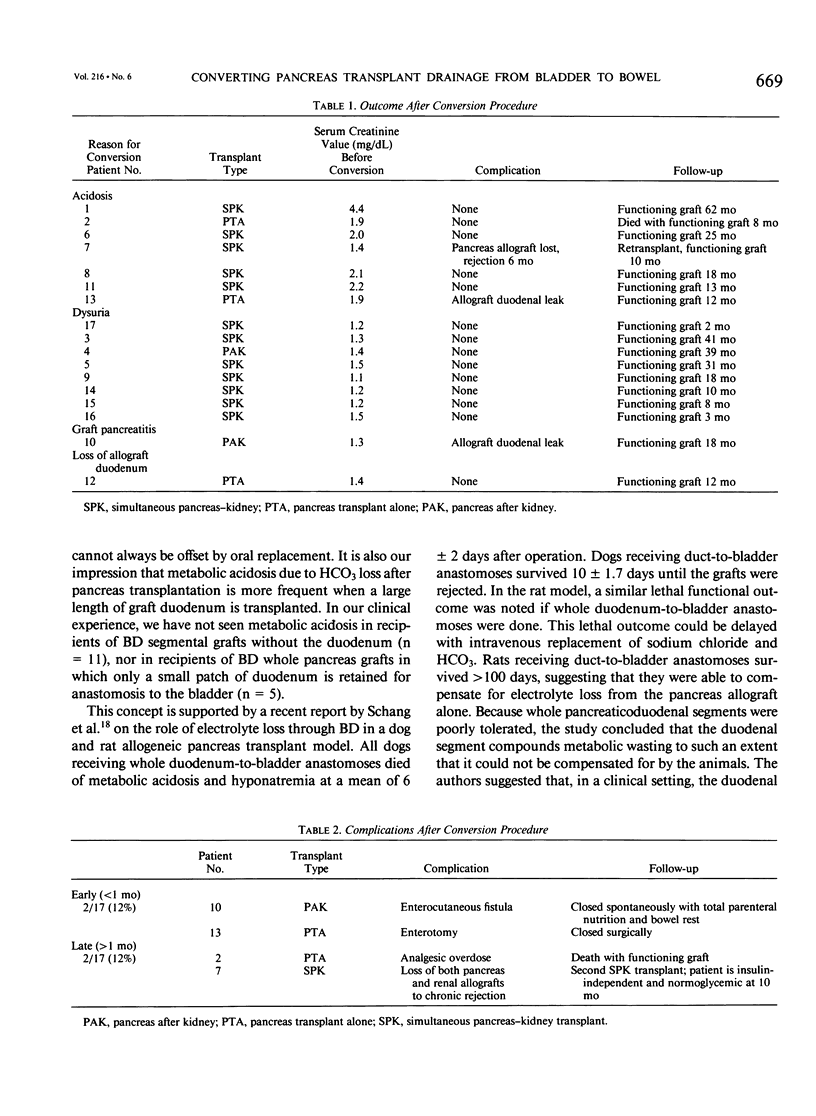
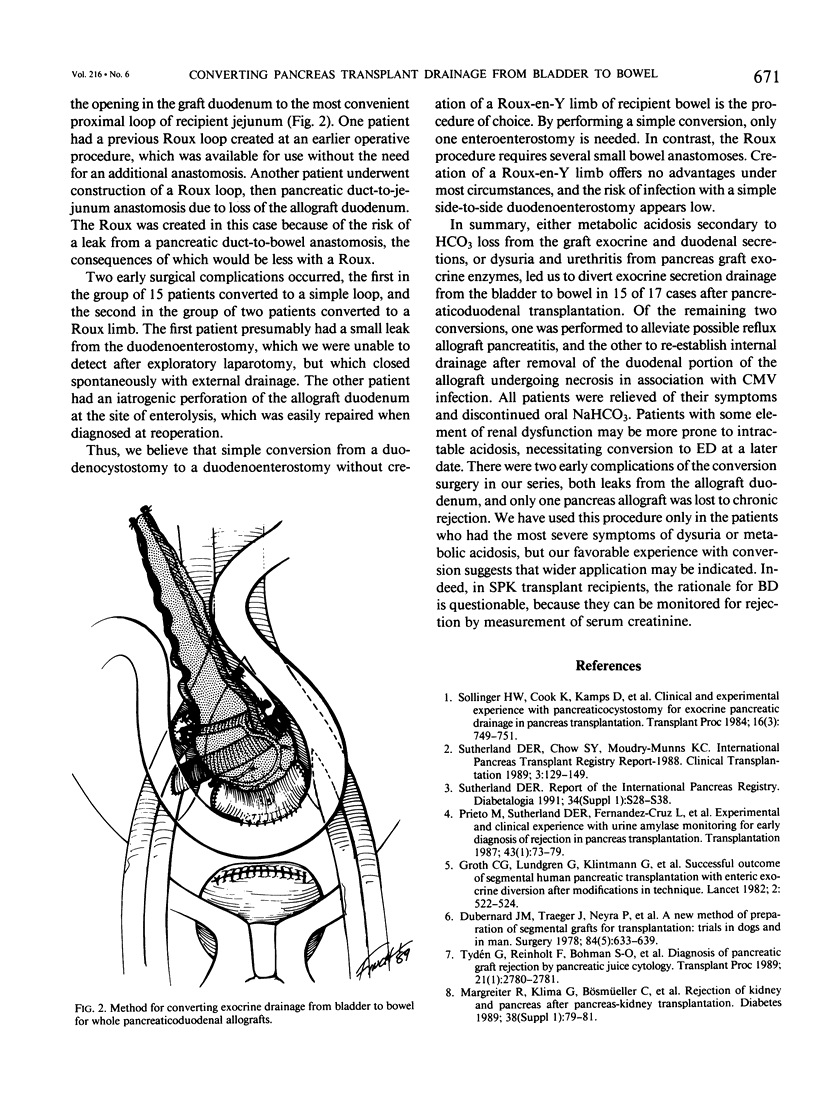
Between September 1984 and August 1991, 265 whole pancreaticoduodenal transplants were done at our institution, with bladder drainage of exocrine secretions through a duodenocystostomy. Seventeen patients subsequently underwent conversion from bladder to enteric drainage at 2 to 64 months after transplant. Eight conversion procedures were done to correct chronic intractable metabolic acidosis due to bicarbonate loss from the allograft: seven to alleviate severe dysuria, presumed secondary to the action of graft enzymes on uroepithelium; one to prevent recurrent allograft pancreatitis, presumed secondary to back pressure from the bladder; and one because of graft duodenectomy for severe cytomegalovirus duodenitis with perforation. None were done to correct technical complications from the initial transplant operation. The conversions were done by dividing the graft duodenocystostomy, then re-establishing drainage through a graft duodenal-recipient jejunal anastomosis. A simple loop of recipient jejunum was used for the duodenojejunostomy in 15 cases, and a Roux limb in two. One of those two cases had a previously created Roux limb that was available for use. The other was in the patient who underwent graft duodenectomy and subsequent mucosa-to-mucosa anastomosis of the pancreatic duct to a newly created Roux limb of jejunum. All patients experienced relief of their symptoms after operation. Two patients had surgical complications (12%), an enterotomy in one case, which was closed operatively, and an enterocutaneous fistula in the other case, which healed spontaneously with bowel rest and parenteral nutrition. The drawback to conversion is loss of urine amylase as a marker for rejection, particularly in recipients of solitary pancreas grafts (n = 5). In recipients of simultaneous pancreas-kidney (SPK) allografts (n = 12), the kidney can still be used to monitor for rejection (two with follow-up < 1 year, 10 with follow-up > 1 year). None of our solitary pancreas recipients, however, have lost graft function (follow-up, 10 to 36 months). The only pancreas allograft loss was in an SPK recipient who also rejected the kidney 6 months after conversion. She received a second SPK transplant with enteric drainage, and is insulin independent and normoglycemic 10 months after retransplantation. Patients converted for metabolic acidosis tended to have impaired renal function (mean creatinine, 2.14 +/- 0.98 mg/dL at time of conversion) due to chronic rejection, progression of native kidney diabetic nephropathy, or cyclosporine toxicity, and possibly could not compensate for bicarbonate loss from the pancreas allograft.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke G. W., Gruessner R., Dunn D. L., Sutherland D. E. Conversion of whole pancreaticoduodenal transplants from bladder to enteric drainage for metabolic acidosis or dysuria. Transplant Proc. 1990 Apr;22(2):651–652. [PubMed] [Google Scholar]
- Dubernard J. M., Traeger J., Neyra P., Touraine J. L., Tranchant D., Blanc-Brunat N. A new method of preparation of segmental pancreatic grafts for transplantation: trials in dogs and in man. Surgery. 1978 Nov;84(5):633–639. [PubMed] [Google Scholar]
- Franco E., Aguiló F., Muñoz J., Rusconi A., Castelao A., Griñ J. M., Cruz O., Torrecilla C., Gil-Vernet A., Serrallach N. Polar artery damage in kidney procurement: incidence and follow-up of 300 renal allografts. Transplant Proc. 1988 Oct;20(5):887–888. [PubMed] [Google Scholar]
- Groth C. G., Collste H., Lundgren G., Wilczek H., Klintmalm G., Ringdén O., Gunnarsson R., Ostman J. Successful outcome of segmental human pancreatic transplantation with enteric exocrine diversion after modifications in technique. Lancet. 1982 Sep 4;2(8297):522–524. doi: 10.1016/s0140-6736(82)90601-8. [DOI] [PubMed] [Google Scholar]
- Isenberg J. I., Selling J. A., Hogan D. L., Koss M. A. Impaired proximal duodenal mucosal bicarbonate secretion in patients with duodenal ulcer. N Engl J Med. 1987 Feb 12;316(7):374–379. doi: 10.1056/NEJM198702123160704. [DOI] [PubMed] [Google Scholar]
- Margreiter R., Klima G., Bösmüller C., Königsrainer A., Schmid T., Steiner E. Rejection of kidney and pancreas after pancreas-kidney transplantation. Diabetes. 1989 Jan;38 (Suppl 1):79–81. doi: 10.2337/diab.38.1.s79. [DOI] [PubMed] [Google Scholar]
- Marshall V. C., Jablonski P., Biguzas M., Howden B. O., Walls K. University of Wisconsin solution for kidney preservation: the impermeant components. Transplant Proc. 1991 Feb;23(1 Pt 1):651–652. [PubMed] [Google Scholar]
- Nghiem D. D., Corry R. J. Technique of simultaneous renal pancreatoduodenal transplantation with urinary drainage of pancreatic secretion. Am J Surg. 1987 Apr;153(4):405–406. doi: 10.1016/0002-9610(87)90588-5. [DOI] [PubMed] [Google Scholar]
- Pescovitz M. D., Dunn D. L., Sutherland D. E. Use of the circular stapler in construction of the duodenoneocystostomy for drainage into the bladder in transplants involving the whole pancreas. Surg Gynecol Obstet. 1989 Aug;169(2):169–171. [PubMed] [Google Scholar]
- Prieto M., Sutherland D. E., Fernandez-Cruz L., Heil J., Najarian J. S. Experimental and clinical experience with urine amylase monitoring for early diagnosis of rejection in pancreas transplantation. Transplantation. 1987 Jan;43(1):73–79. doi: 10.1097/00007890-198701000-00017. [DOI] [PubMed] [Google Scholar]
- Prieto M., Sutherland D. E., Goetz F. C., Rosenberg M. E., Najarian J. S. Pancreas transplant results according to the technique of duct management: bladder versus enteric drainage. Surgery. 1987 Oct;102(4):680–691. [PubMed] [Google Scholar]
- Schang T., Timmermann W., Thiede A., Najarian J. S., Sutherland D. E. Detrimental effects of fluid and electrolyte loss from duodenum in bladder-drained pancreas transplants. Transplant Proc. 1991 Feb;23(1 Pt 2):1617–1618. [PubMed] [Google Scholar]
- Sollinger H. W., Cook K., Kamps D., Glass N. R., Belzer F. O. Clinical and experimental experience with pancreaticocystostomy for exocrine pancreatic drainage in pancreas transplantation. Transplant Proc. 1984 Jun;16(3):749–751. [PubMed] [Google Scholar]
- Sutherland D. E., Dunn D. L., Goetz F. C., Kennedy W., Ramsay R. C., Steffes M. W., Mauer S. M., Gruessner R., Moudry-Munns K. C., Morel P. A 10-year experience with 290 pancreas transplants at a single institution. Ann Surg. 1989 Sep;210(3):274–288. doi: 10.1097/00000658-198909000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. E., Kendall D. M., Moudry K. C., Navarro X., Kennedy W. R., Ramsay R. C., Steffes M. W., Mauer S. M., Goetz F. C., Dunn D. L. Pancreas transplantation in nonuremic, type I diabetic recipients. Surgery. 1988 Aug;104(2):453–464. [PubMed] [Google Scholar]
- Sutherland D. E. Report from the International Pancreas Transplant Registry. Diabetologia. 1991 Aug;34 (Suppl 1):S28–S39. doi: 10.1007/BF00587615. [DOI] [PubMed] [Google Scholar]
- Tom W. W., Munda R., First M. R., Alexander J. W. Autodigestion of the glans penis and urethra by activated transplant pancreatic exocrine enzymes. Surgery. 1987 Jul;102(1):99–101. [PubMed] [Google Scholar]
- Tydén G., Reinholt F., Bohman S. O., Brattström C., Tibell A., Groth C. G. Diagnosis of pancreatic graft rejection by pancreatic juice cytology. Transplant Proc. 1989 Feb;21(1 Pt 3):2780–2781. [PubMed] [Google Scholar]


