Abstract
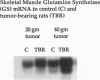
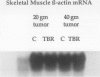
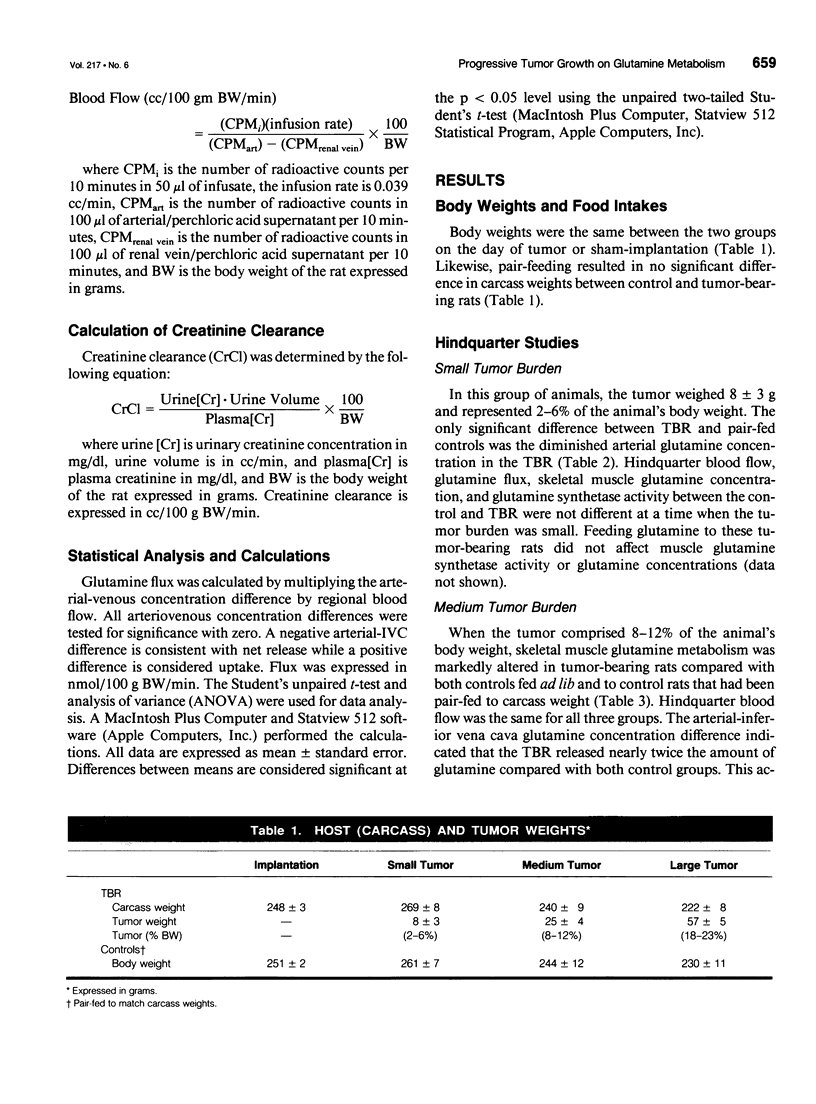
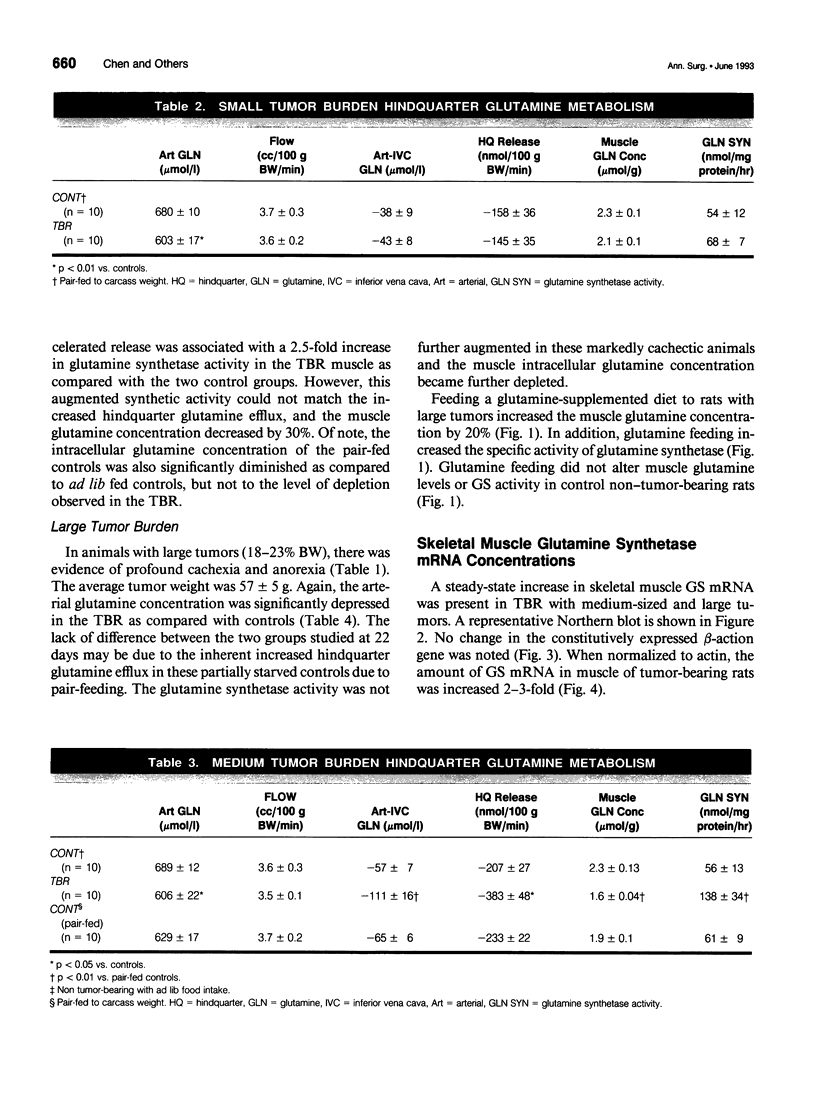
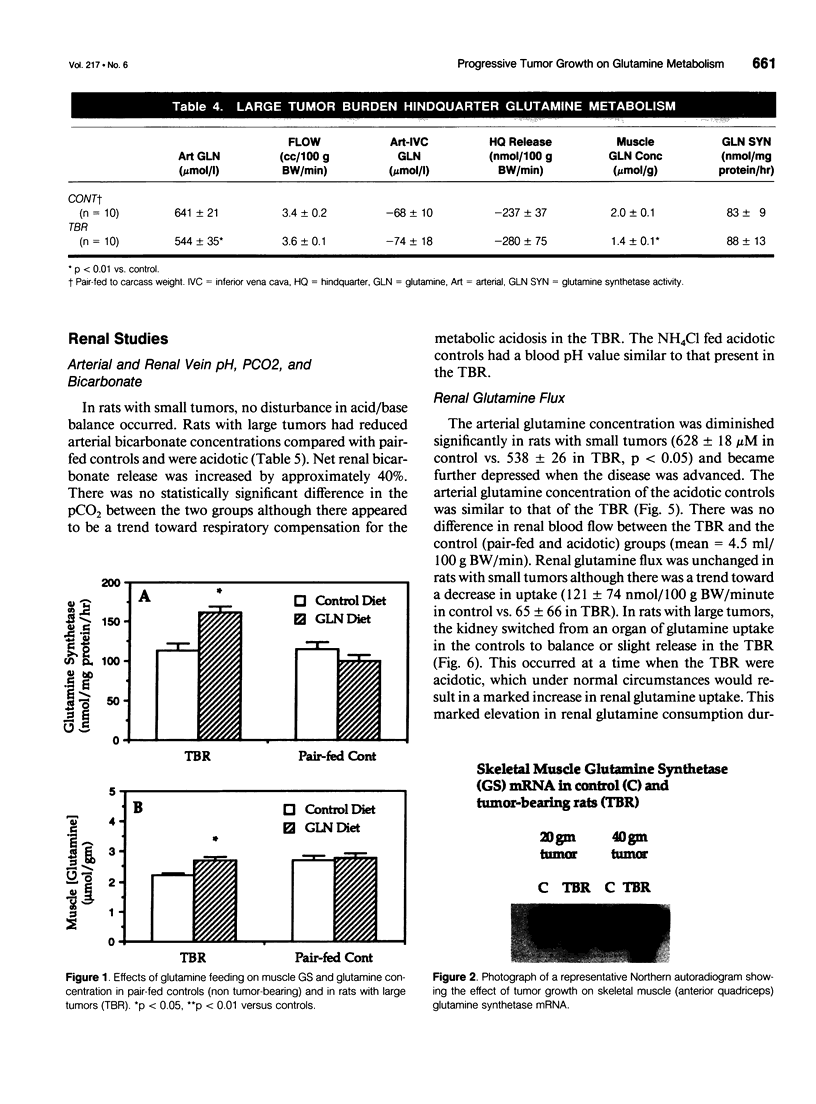
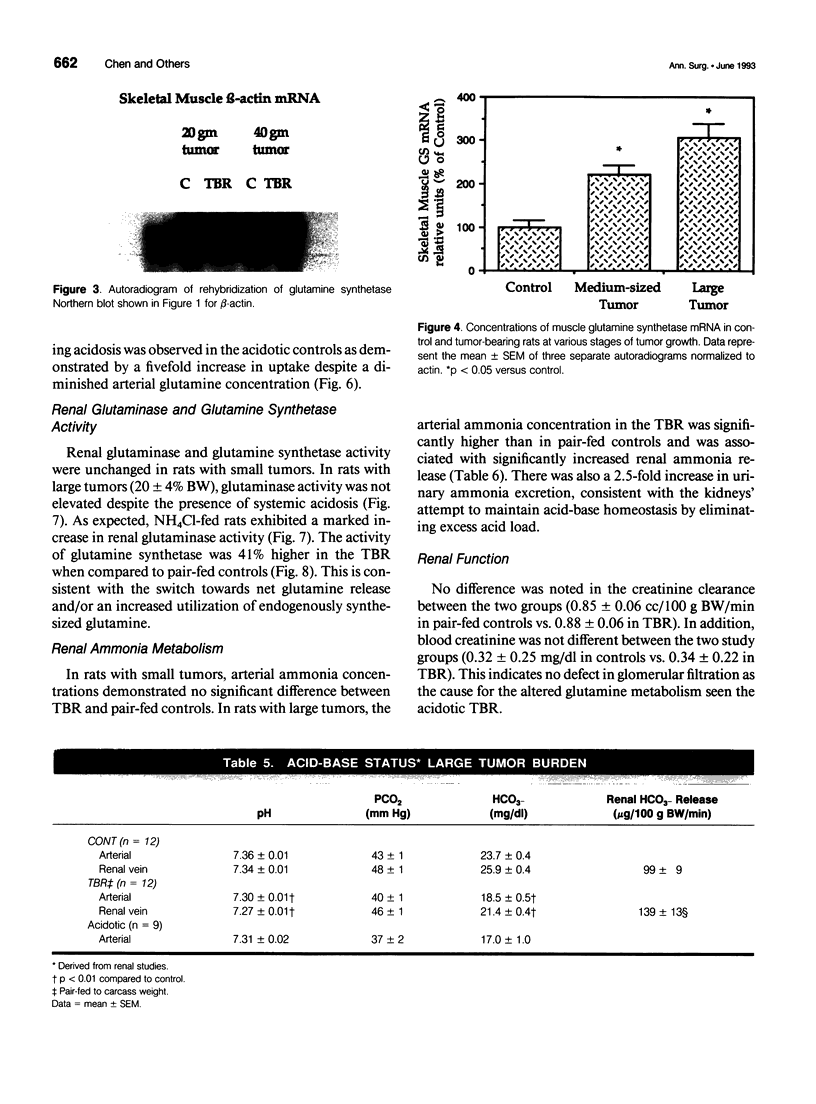
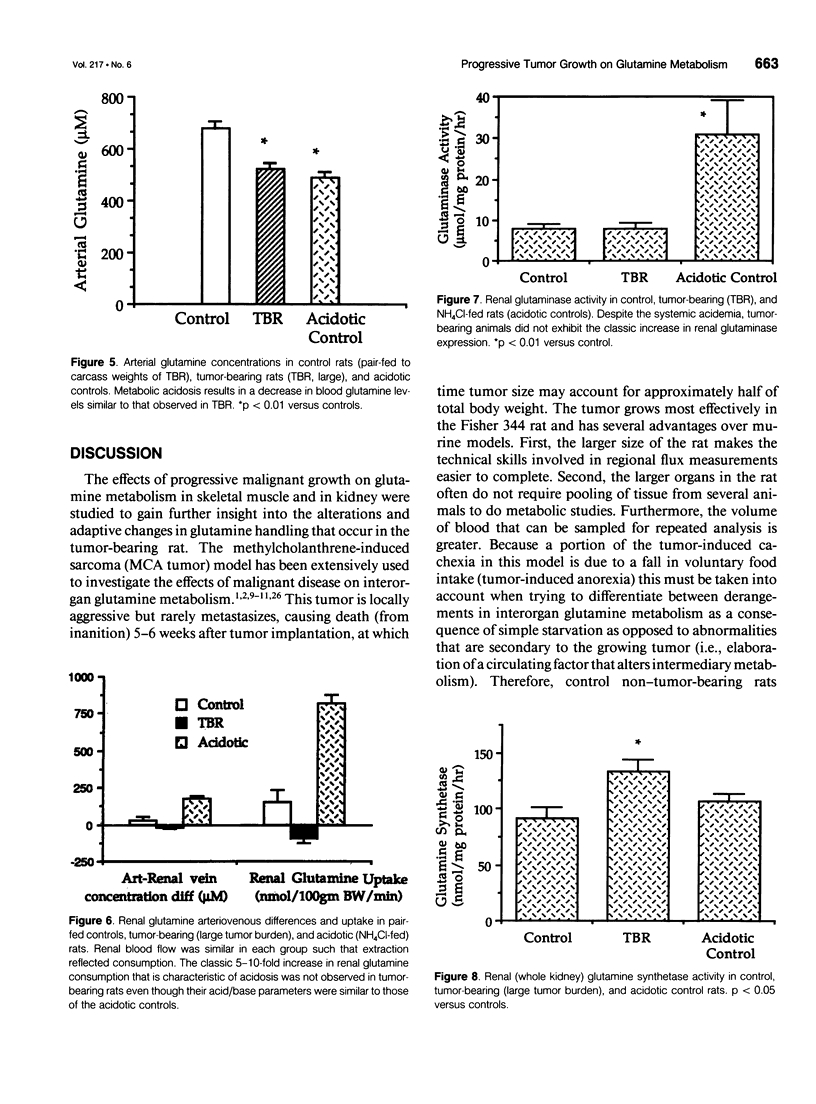
OBJECTIVE: The effects of progressive malignant growth on glutamine metabolism in skeletal muscle and in kidney were investigated. SUMMARY BACKGROUND DATA: Fast-growing tumors consume considerable quantities of glutamine and lead to a decrease in circulating glutamine concentrations. METHODS: Experiments were performed at various stages of tumor growth in rats implanted subcutaneously with the non-metastasizing methylcholanthrene-induced (MCA) fibrosarcoma and in pair-fed non tumor-bearing controls. RESULTS: Tumor growth stimulated a twofold increase in hindquarter (muscle) glutamine release, which was not due to an increase in blood flow, but rather to a doubling in the fractional release rate. Consequently, a progressive decrease in skeletal muscle glutamine concentrations was observed over time. Simultaneously, the activity of glutamine synthetase (GS), the principal enzyme of de novo glutamine biosynthesis, increased more than twofold. This increase in muscle GS activity was accompanied by an increase in GS mRNA but the augmentation in GS expression apparently could not match the increased rate of efflux since muscle depletion developed. In rats with large tumors and severe glutamine depletion, GS activity was not elevated. Glutamine feeding increased muscle glutamine concentrations and glutamine synthetase specific activity. Although tumor growth led to the development of mild systemic acidemia, the classic renal adaptations normally observed, i.e., elevated glutaminase activity and accelerated renal glutamine utilization, were not present in acidotic tumor-bearing rats. Instead, renal GS activity was increased in tumor-bearing animals and ammoniagenesis was enhanced, in spite of a reduction in net renal glutamine uptake. CONCLUSIONS: These data suggest that marked alterations in muscle and renal glutamine handling occur in the host with cancer; the enhanced muscle glutamine release in conjunction with no increase in renal consumption is consistent with increased glutamine uptake in other organs, most likely the tumor itself and the liver.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardawi M. S. Skeletal muscle glutamine production in thermally injured rats. Clin Sci (Lond) 1988 Feb;74(2):165–172. doi: 10.1042/cs0740165. [DOI] [PubMed] [Google Scholar]
- Austgen T. R., Chakrabarti R., Chen M. K., Souba W. W. Adaptive regulation in skeletal muscle glutamine metabolism in endotoxin-treated rats. J Trauma. 1992 May;32(5):600–607. doi: 10.1097/00005373-199205000-00011. [DOI] [PubMed] [Google Scholar]
- Austgen T. R., Chen M. K., Moore W., Souba W. W. Endotoxin and renal glutamine metabolism. Arch Surg. 1991 Jan;126(1):23–27. doi: 10.1001/archsurg.1991.01410250027003. [DOI] [PubMed] [Google Scholar]
- Austgen T. R., Dudrick P. S., Sitren H., Bland K. I., Copeland E., Souba W. W. The effects of glutamine-enriched total parenteral nutrition on tumor growth and host tissues. Ann Surg. 1992 Feb;215(2):107–113. doi: 10.1097/00000658-199202000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch H. B., Chan A. W., Alvey T. R., Lowry O. H. Localization of glutamine accumulation and tubular reabsorption in rat nephron. Kidney Int. 1978 Nov;14(5):406–413. doi: 10.1038/ki.1978.145. [DOI] [PubMed] [Google Scholar]
- Chen M. K., Salloum R. M., Austgen T. R., Bland J. B., Bland K. I., Copeland E. M., 3rd, Souba W. W. Tumor regulation of hepatic glutamine metabolism. JPEN J Parenter Enteral Nutr. 1991 Mar-Apr;15(2):159–164. doi: 10.1177/0148607191015002159. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischer J. E., Chance W. T. Total parenteral nutrition, glutamine, and tumor growth. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):86S–89S. doi: 10.1177/0148607190014004101. [DOI] [PubMed] [Google Scholar]
- Fox A. D., Kripke S. A., De Paula J., Berman J. M., Settle R. G., Rombeau J. L. Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr. 1988 Jul-Aug;12(4):325–331. doi: 10.1177/0148607188012004325. [DOI] [PubMed] [Google Scholar]
- Hammarqvist F., Wernerman J., Ali R., von der Decken A., Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg. 1989 Apr;209(4):455–461. doi: 10.1097/00000658-198904000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimberg V. S., Salloum R. M., Kasper M., Plumley D. A., Dolson D. J., Hautamaki R. D., Mendenhall W. R., Bova F. C., Bland K. I., Copeland E. M., 3rd Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch Surg. 1990 Aug;125(8):1040–1045. doi: 10.1001/archsurg.1990.01410200104017. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Souba W. W., Dolson D. J., Salloum R. M., Hautamaki R. D., Plumley D. A., Mendenhall W. M., Bova F. J., Khan S. R., Hackett R. L. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990 Jul 1;66(1):62–68. doi: 10.1002/1097-0142(19900701)66:1<62::aid-cncr2820660113>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Souba W. W., Salloum R. M., Plumley D. A., Cohen F. S., Dolson D. J., Bland K. I., Copeland E. M., 3rd Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J Surg Res. 1990 Apr;48(4):319–323. doi: 10.1016/0022-4804(90)90066-b. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Horowitz M. L., Friedell G. H. The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res. 1969 Mar;29(3):669–680. [PubMed] [Google Scholar]
- Kovacević Z., Morris H. P. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972 Feb;32(2):326–333. [PubMed] [Google Scholar]
- Lemieux G., Baverel G., Vinay P., Wadoux P. Glutamine synthetase and glutamyltransferase in the kidney of man, dog, and rat. Am J Physiol. 1976 Oct;231(4):1068–1073. doi: 10.1152/ajplegacy.1976.231.4.1068. [DOI] [PubMed] [Google Scholar]
- Linder-Horowitz M., Knox W. E., Morris H. P. Glutaminase activities and growth rates of rat hepatomas. Cancer Res. 1969 Jun;29(6):1195–1199. [PubMed] [Google Scholar]
- Max S. R., Mill J., Mearow K., Konagaya M., Konagaya Y., Thomas J. W., Banner C., Vitković L. Dexamethasone regulates glutamine synthetase expression in rat skeletal muscles. Am J Physiol. 1988 Sep;255(3 Pt 1):E397–E402. doi: 10.1152/ajpendo.1988.255.3.E397. [DOI] [PubMed] [Google Scholar]
- Max S. R., Thomas J. W., Banner C., Vitkovic L., Konagaya M., Konagaya Y. Glucocorticoid receptor-mediated induction of glutamine synthetase in skeletal muscle cells in vitro. Endocrinology. 1987 Mar;120(3):1179–1183. doi: 10.1210/endo-120-3-1179. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Jepson M. M., Omer A. Muscle glutamine concentration and protein turnover in vivo in malnutrition and in endotoxemia. Metabolism. 1989 Aug;38(8 Suppl 1):6–13. doi: 10.1016/0026-0495(89)90132-7. [DOI] [PubMed] [Google Scholar]
- OWEN E. E., ROBINSON R. R. Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest. 1963 Feb;42:263–276. doi: 10.1172/JCI104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacitti A. J., Chen M. K., Bland K. I., Copeland E. M., Souba W. W. Mechanisms of accelerated hepatic glutamine efflux in the tumour-bearing rat. Surg Oncol. 1992 Apr;1(2):173–182. doi: 10.1016/0960-7404(92)90031-f. [DOI] [PubMed] [Google Scholar]
- Pinkus L. M., Windmueller H. G. Phosphate-dependent glutaminase of small intestine: localization and role in intestinal glutamine metabolism. Arch Biochem Biophys. 1977 Aug;182(2):506–517. doi: 10.1016/0003-9861(77)90531-8. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979 Apr 25;254(8):2669–2676. [PubMed] [Google Scholar]
- Rennie M. J., MacLennan P. A., Hundal H. S., Weryk B., Smith K., Taylor P. M., Egan C., Watt P. W. Skeletal muscle glutamine transport, intramuscular glutamine concentration, and muscle-protein turnover. Metabolism. 1989 Aug;38(8 Suppl 1):47–51. doi: 10.1016/0026-0495(89)90140-6. [DOI] [PubMed] [Google Scholar]
- Scheltinga M. R., Young L. S., Benfell K., Bye R. L., Ziegler T. R., Santos A. A., Antin J. H., Schloerb P. R., Wilmore D. W. Glutamine-enriched intravenous feedings attenuate extracellular fluid expansion after a standard stress. Ann Surg. 1991 Oct;214(4):385–395. doi: 10.1097/00000658-199110000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröck H., Goldstein L. Interorgan relationships for glutamine metabolism in normal and acidotic rats. Am J Physiol. 1981 May;240(5):E519–E525. doi: 10.1152/ajpendo.1981.240.5.E519. [DOI] [PubMed] [Google Scholar]
- Souba W. W., Strebel F. R., Bull J. M., Copeland E. M., Teagtmeyer H., Cleary K. Interorgan glutamine metabolism in the tumor-bearing rat. J Surg Res. 1988 Jun;44(6):720–726. doi: 10.1016/0022-4804(88)90106-0. [DOI] [PubMed] [Google Scholar]
- Squires E. J., Hall D. E., Brosnan J. T. Arteriovenous differences for amino acids and lactate across kidneys of normal and acidotic rats. Biochem J. 1976 Oct 15;160(1):125–128. doi: 10.1042/bj1600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle P., Zander J., Mertes N., Albers S., Puchstein C., Lawin P., Fürst P. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989 Feb 4;1(8632):231–233. doi: 10.1016/s0140-6736(89)91254-3. [DOI] [PubMed] [Google Scholar]
- Welbourne T. C., Phromphetcharat V., Givens G., Joshi S. Regulation of interorganal glutamine flow in metabolic acidosis. Am J Physiol. 1986 Apr;250(4 Pt 1):E457–E463. doi: 10.1152/ajpendo.1986.250.4.E457. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Aikawa T., Matsuaka H., Ishikawa E. Relative uptake of plasma amino acids by fetal and tumor tissues. Metabolism. 1974 Nov;23(11):1017–1022. doi: 10.1016/0026-0495(74)90068-7. [DOI] [PubMed] [Google Scholar]
- Ziegler T. R., Young L. S., Benfell K., Scheltinga M., Hortos K., Bye R., Morrow F. D., Jacobs D. O., Smith R. J., Antin J. H. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med. 1992 May 15;116(10):821–828. doi: 10.7326/0003-4819-116-10-821. [DOI] [PubMed] [Google Scholar]