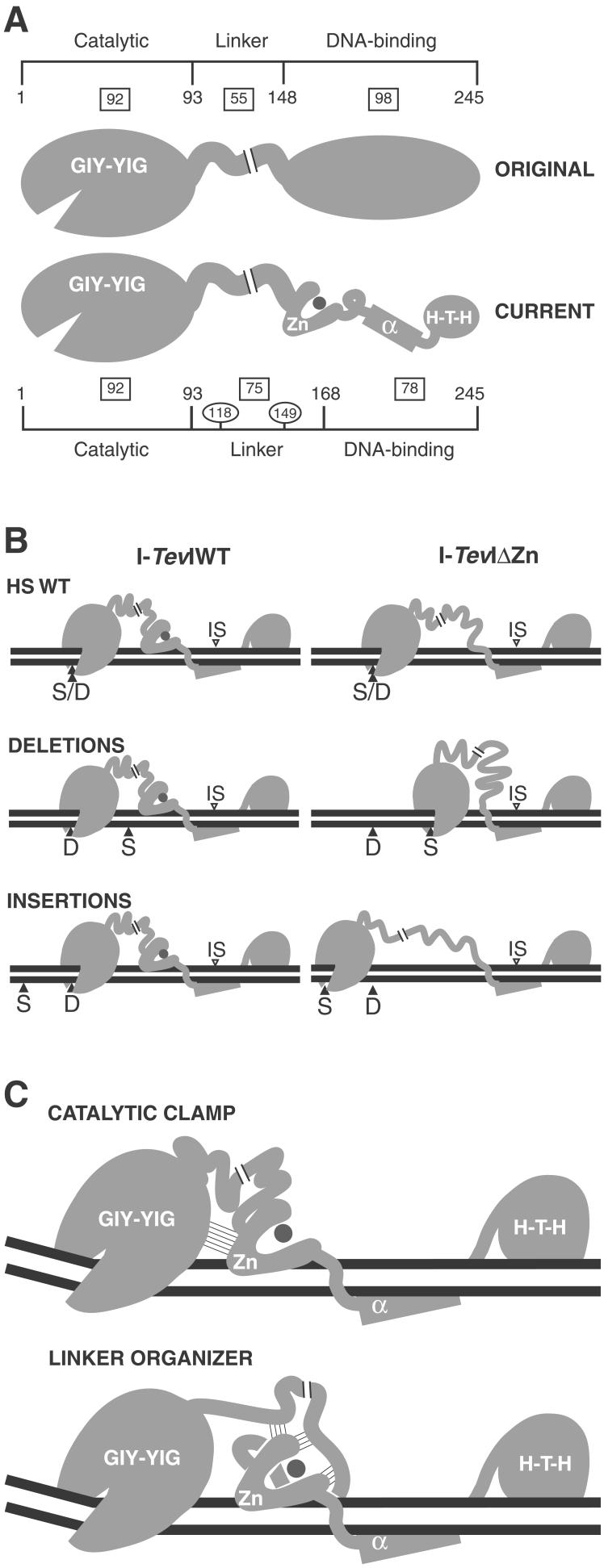
Figure 6.
Models for the role of the zinc finger in I-TevI. The regions of I-TevI were: GIY-YIG, catalytic domain; Zn, zinc finger; α, α-helix; H-T-H, helix–turn–helix. (A) Domains of I-TevI. Original and current models are shown. Boxes indicate the number of residues in each segment of the protein. Residue numbers indicate domain boundaries. Numbers in ovals demarcate the three segments of the linker. (B) Distance-constraining activity of the zinc finger. At large deletions and insertions, wild-type I-TevI defaults to cleave at wild-type distance (Left), whereas ΔZn cleaves predominantly at native cleavage sequence (Right). Labels are as in Fig. 5. (C) Models for directing catalytic domain to a fixed distance. Thin lines depict hypothetical interactions. The catalytic-clamp model shows interactions between the zinc finger and the catalytic domain, whereas the linker-organizer model shows the zinc finger interacting with the linker, to position the catalytic center of I-TevI at the CS. In both models the zinc finger is proposed to be anchored on the DNA, and in both cases, protein–protein interactions limit flexibility and therefore promote distance-specific cleavage by the sequence-tolerant catalytic domain.