Abstract
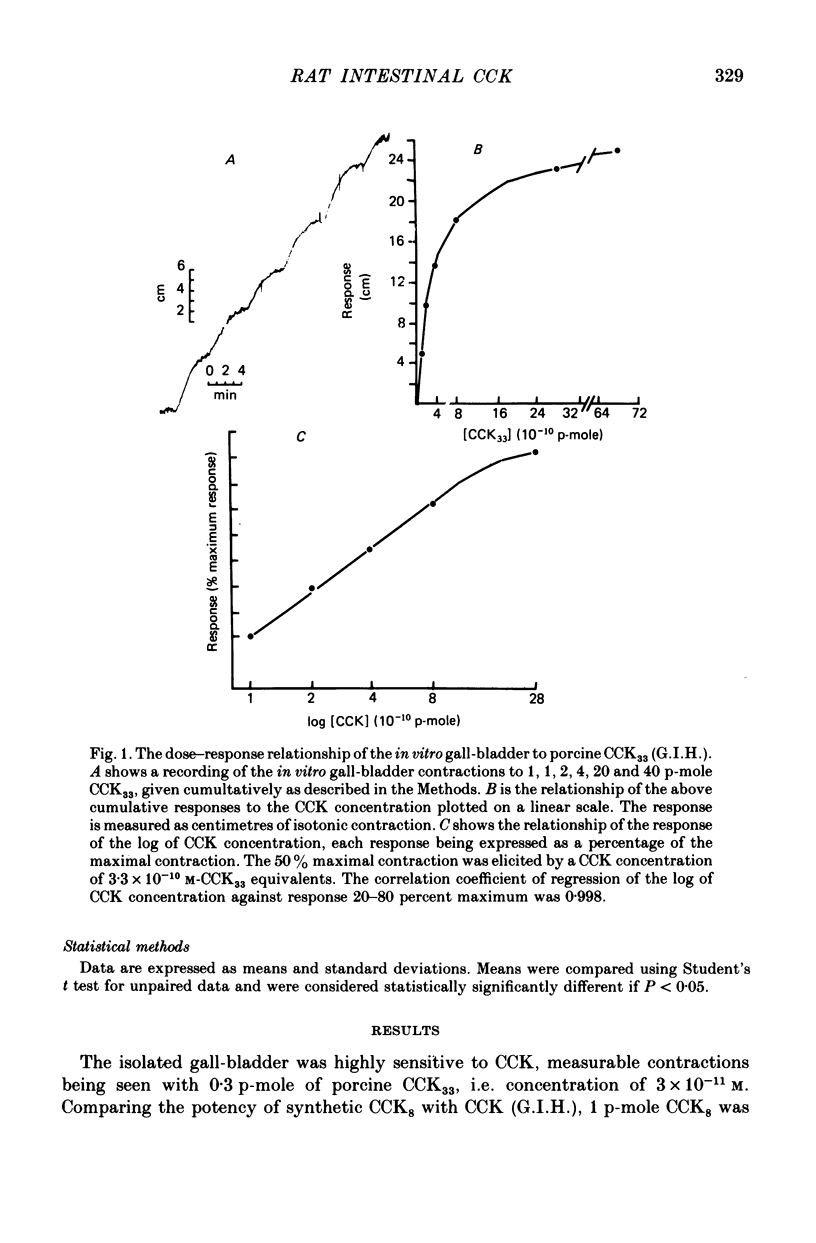
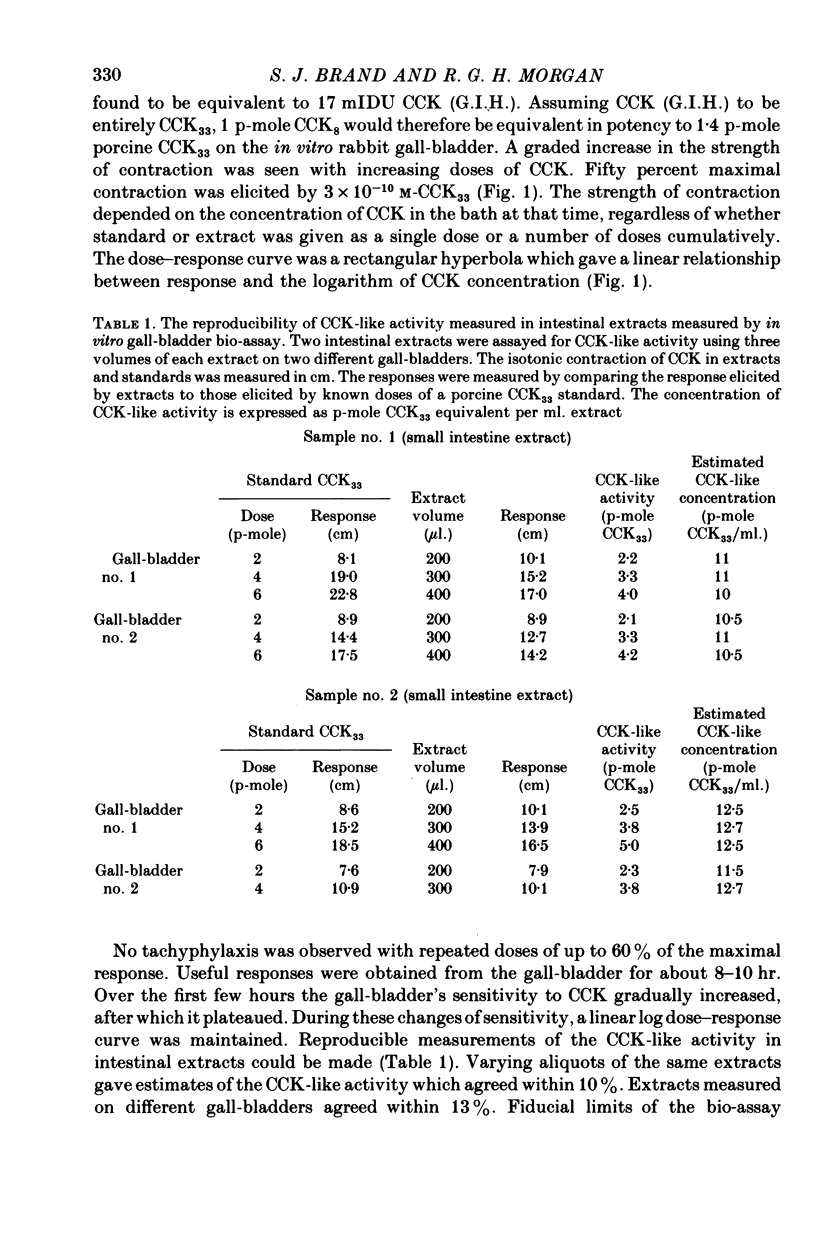
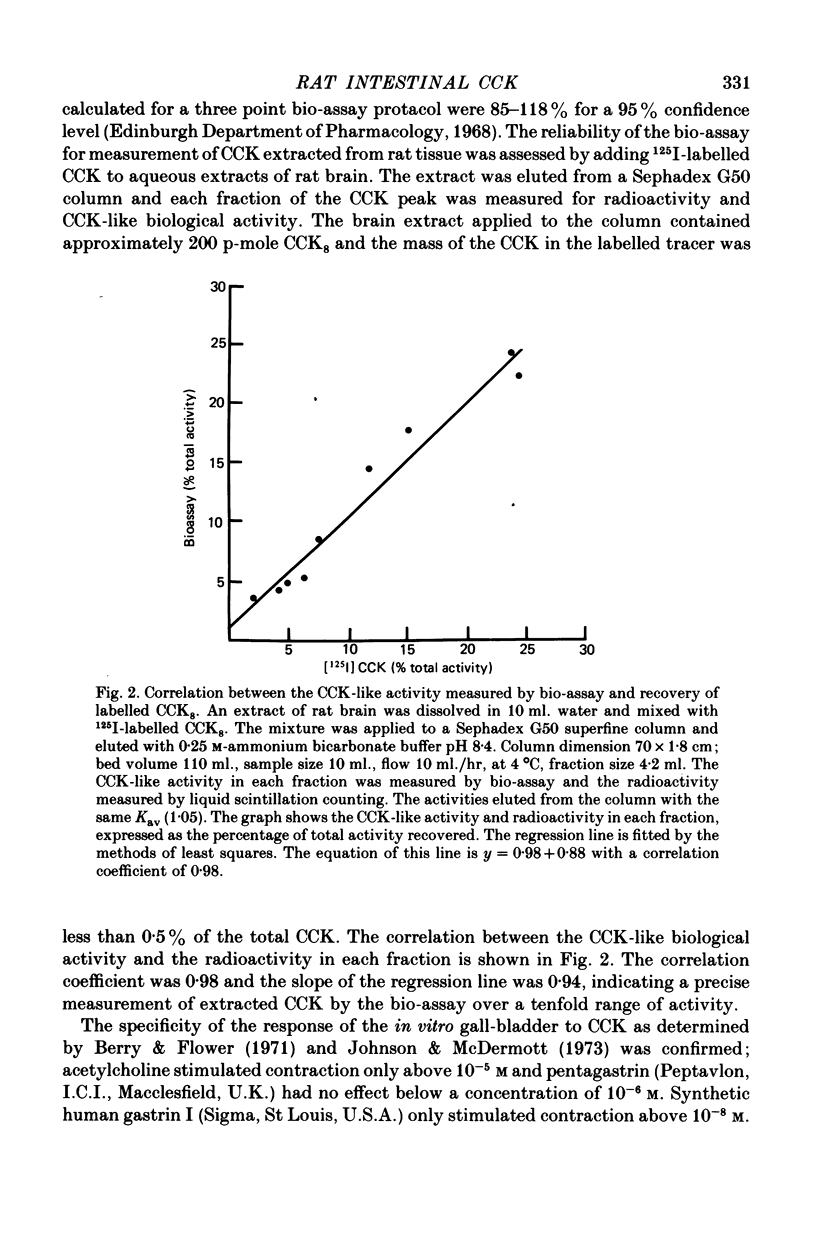
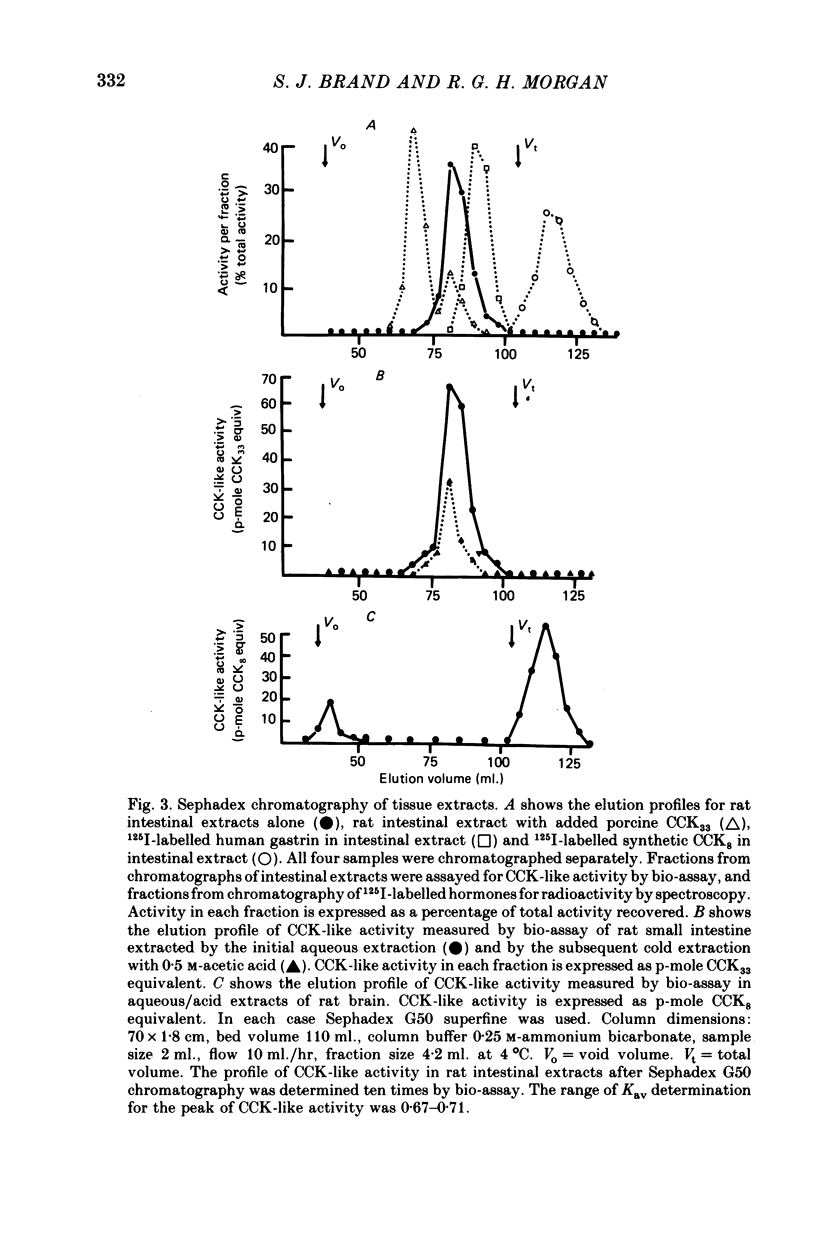
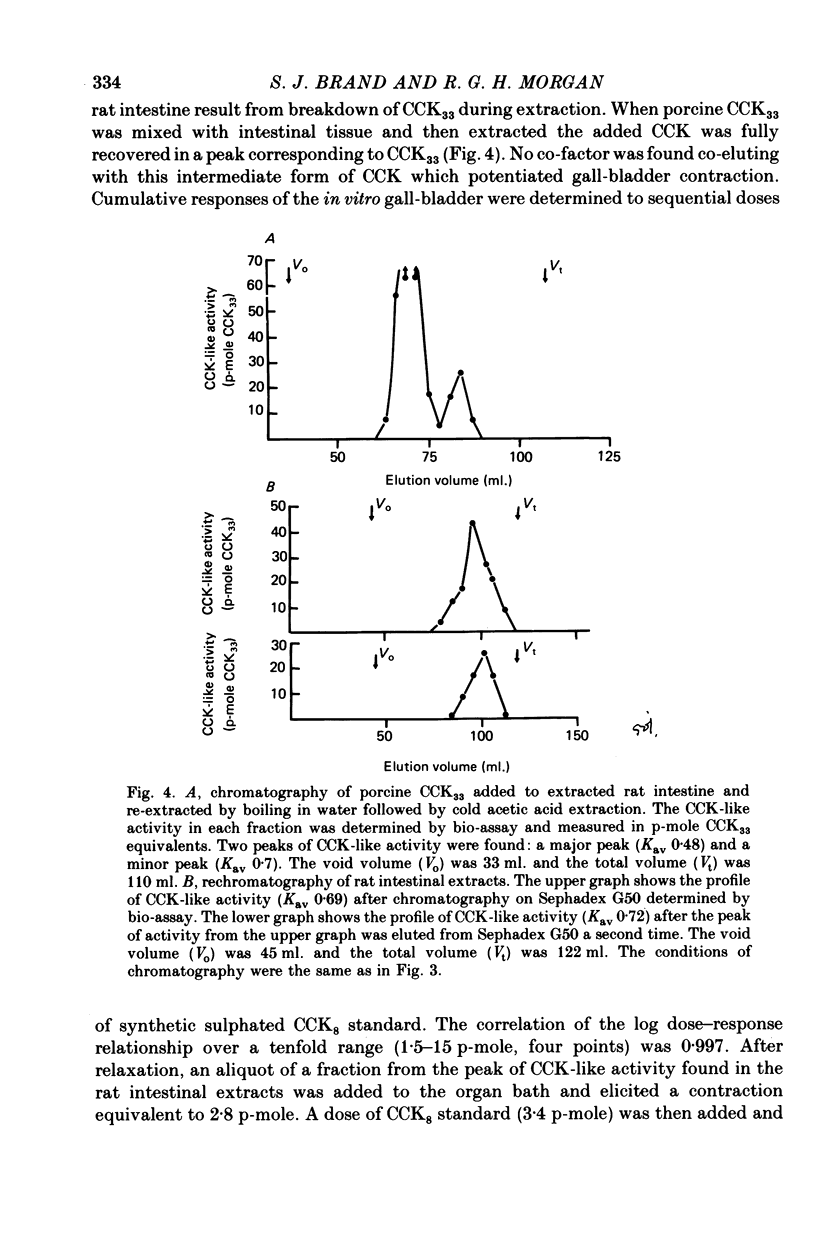
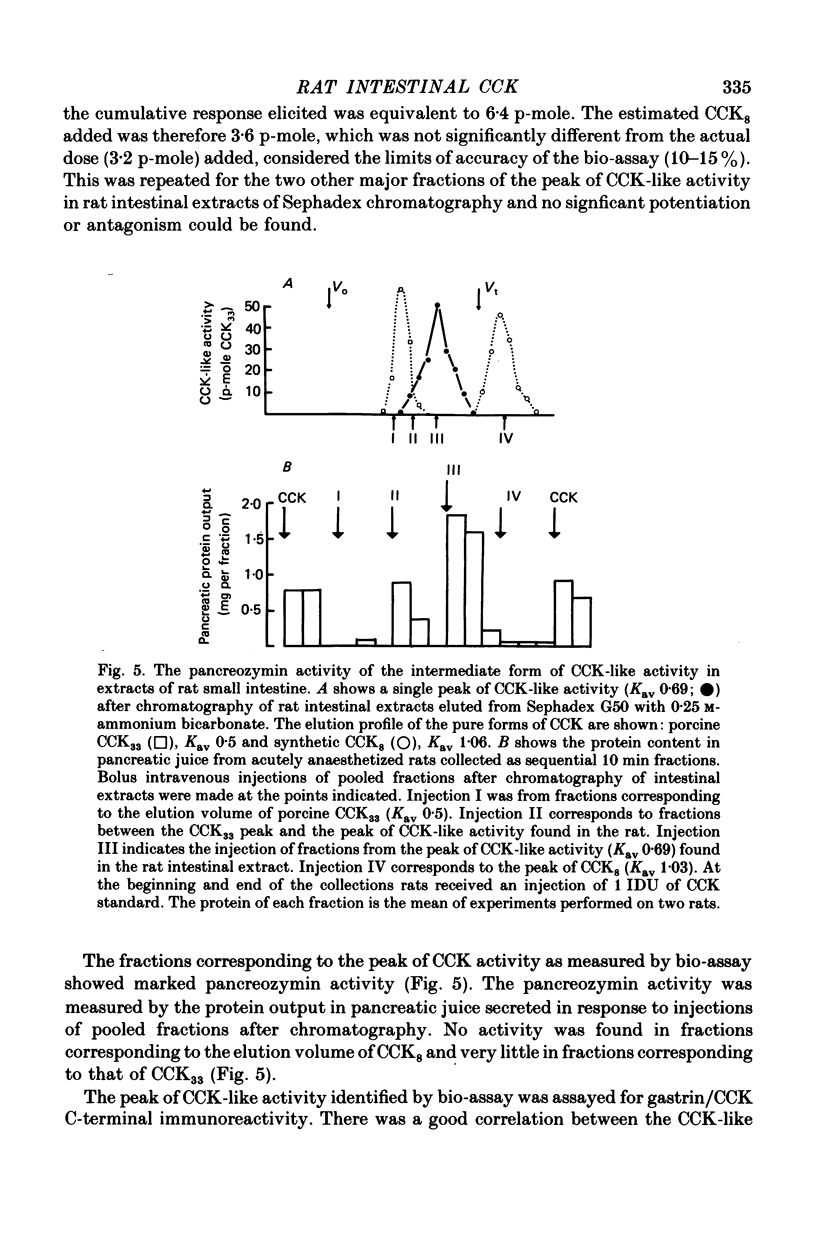
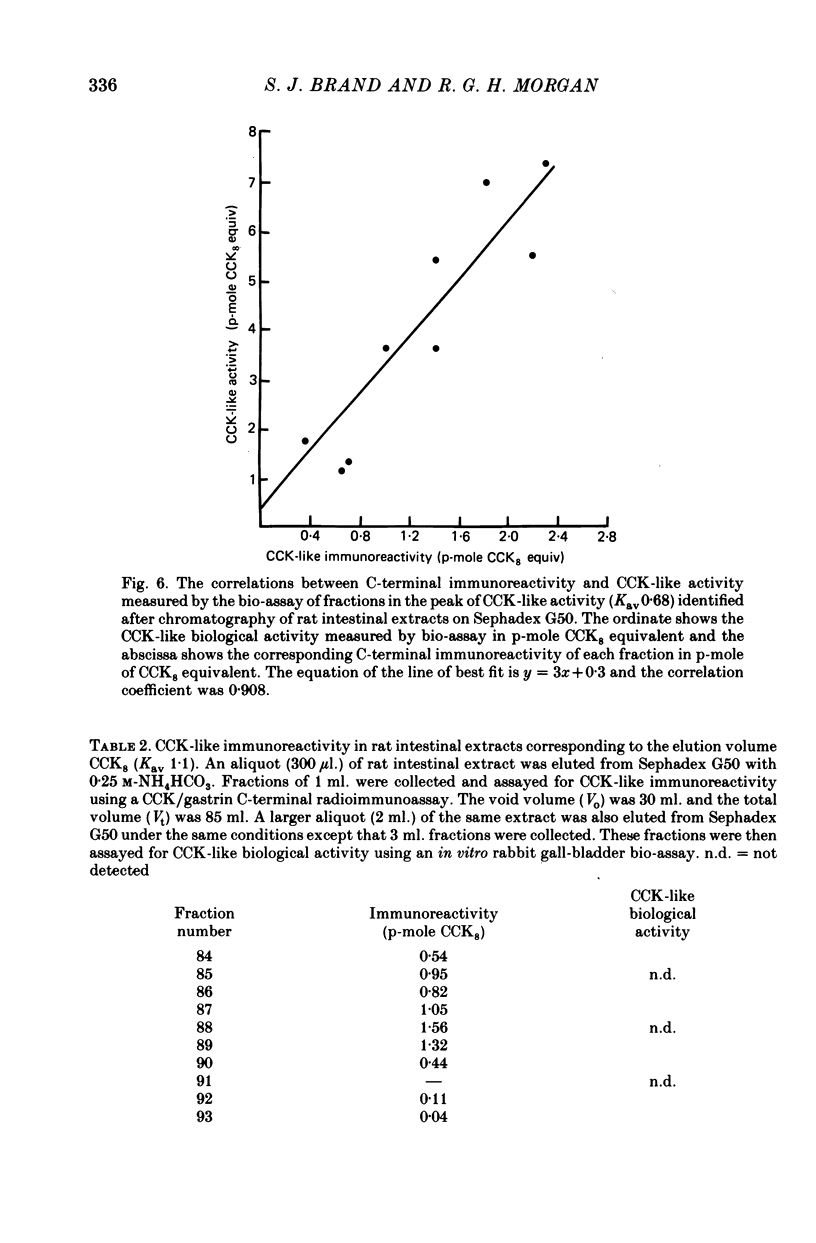
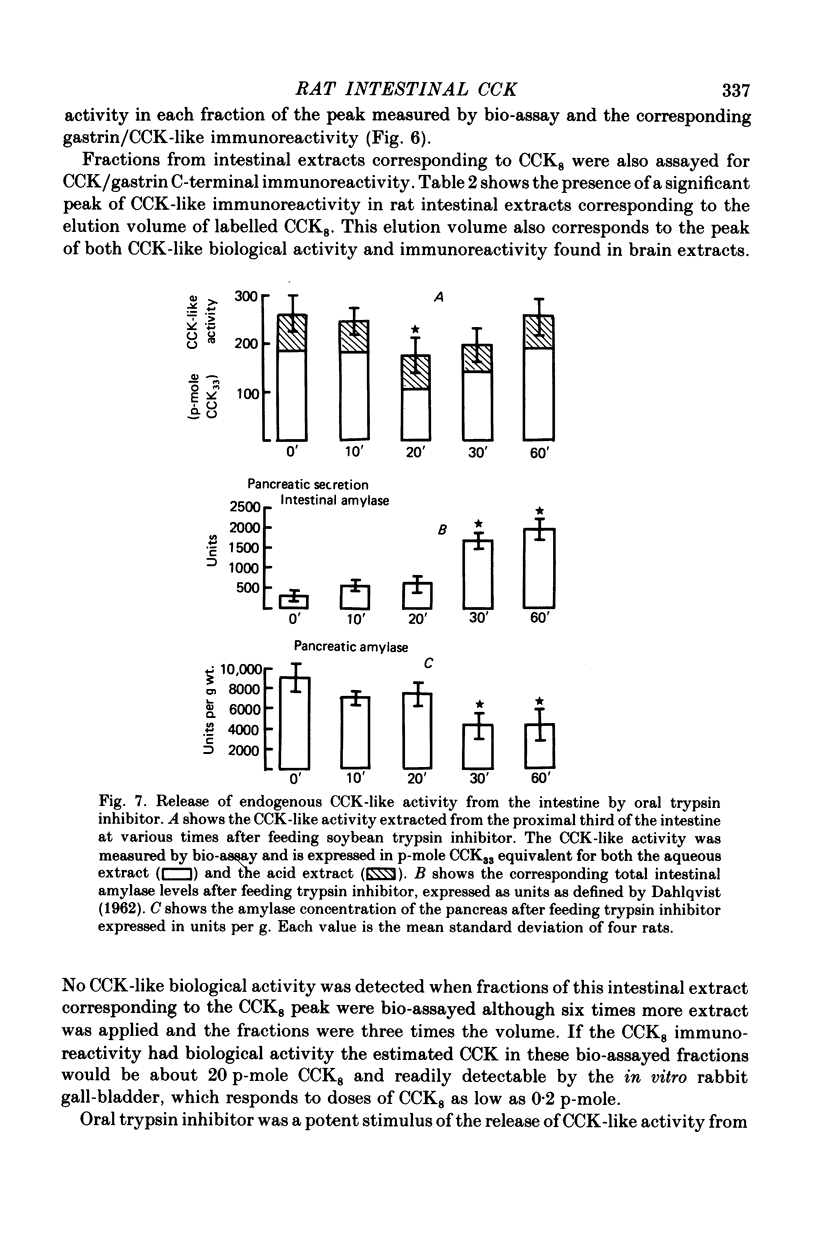
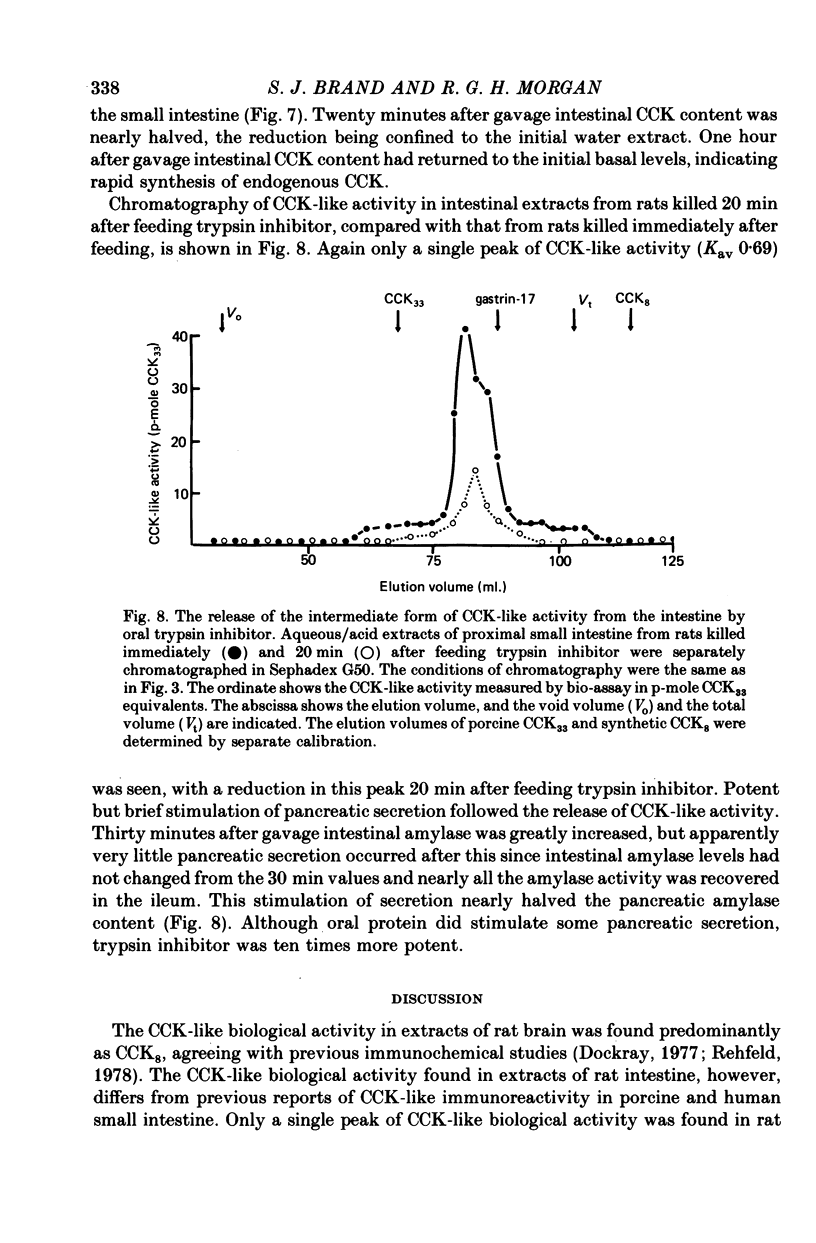
The distribution, molecular form and release of cholecystokinin (CCK)-like activity in extracts of rat small intestine was studied with an in vitro gall-bladder bio-assay. In contrast to the reported heterogeneity of CCK-like immunoreactivity in the intestine, only a single molecular form of CCK-like activity was detected using the bio-assay. 2. The CCK-like activity eluted from Sephadex G50 with a Kav of 0.69, after the triacontriapeptide of cholecystokinin (CCK33) and before cholecystokinin octapeptide 2500, may represent the 22 amino acid peptide of CCK (CCK22). The bio-assay peak of CCK-like activity had pancreozymin activity and CCK/gastrin C terminal immunoreactivity. The CCK-like activity weas readily extracted from the small intestine at neutral pH, but subsequent treatment with cold 0.5 M-acetic acid extracted further CCK-like activity of the same molecular form as that recovered under neutral conditions. 3. The bio-assay detected no CCK-like activity, nor was pancreozymin-like activity found in fractions corresponding to CCK33 or CCK8 after Sephadex G50 chromatography of rat intestinal extracts. 4. Oral trypsin inhibitor was a potent stimulus for the release of CCK-like activity from the upper small intestine of the rat. After oral trypsin inhibitor release, CCK-like activity was rapidly resynthesized.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amer M. S. Studies with cholecystokinin in vitro. 3. Mechanism of the effect on the isolated rabbit gall bladder strips. J Pharmacol Exp Ther. 1972 Dec;183(3):527–534. [PubMed] [Google Scholar]
- Amer M. S. Studies with cholecystokinin. II. Cholecystokinetic potency of porcine gastrins I and II and related peptides in three systems. Endocrinology. 1969 May;84(5):1277–1281. doi: 10.1210/endo-84-5-1277. [DOI] [PubMed] [Google Scholar]
- Berry H., Flower R. J. The assay of endogenous cholecystokinin and factors influencing its release in the dog and cat. Gastroenterology. 1971 Mar;60(3):409–420. [PubMed] [Google Scholar]
- DAHLQVIST A. A method for the determination of amylase in intestinal content. Scand J Clin Lab Invest. 1962;14:145–151. doi: 10.3109/00365516209079686. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Cholecystokinins in rat cerebral cortex: identification, purification and characterization by immunochemical methods. Brain Res. 1980 Apr 21;188(1):155–165. doi: 10.1016/0006-8993(80)90564-8. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunoreactive component resembling cholecystokinin octapeptide in intestine. Nature. 1977 Nov 24;270(5635):359–361. doi: 10.1038/270359a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. The action of scretin, cholecystokinin-pancreozymin and caerulein on pancreatic secretion in the rat. J Physiol. 1972 Sep;225(3):679–692. doi: 10.1113/jphysiol.1972.sp009963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltermann N. R., Rehfeld J. F., Roigaard-Petersen H. In vivo biosynthesis of cholecystokinin in rat cerebral cortex. J Biol Chem. 1980 Jul 10;255(13):6181–6185. [PubMed] [Google Scholar]
- Green G. M., Lyman R. L. Feedback regulation of pancreatic enzyme secretion as a mechanism for trypsin inhibitor-induced hypersecretion in rats. Proc Soc Exp Biol Med. 1972 May;140(1):6–12. doi: 10.3181/00379727-140-36384. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hansky J., Ho P. Cholecystokinin-like peptides in brain and intestine of obese-hyperglycaemic mice. Aust J Exp Biol Med Sci. 1979 Dec;57(6):575–579. doi: 10.1038/icb.1979.59. [DOI] [PubMed] [Google Scholar]
- Johnson A. G., McDermott S. J. Sensitive bioassay of cholecystokinin in human serum. Lancet. 1973 Sep 15;2(7829):589–591. doi: 10.1016/s0140-6736(73)92416-1. [DOI] [PubMed] [Google Scholar]
- Khayambashi H., Lyman R. L. Secretion of rat pancreas perfused with plasma from rats fed soybean trypsin inhibitor. Am J Physiol. 1969 Sep;217(3):646–651. doi: 10.1152/ajplegacy.1969.217.3.646. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Håkanson R., Rehfeld J. F., Stadil F., Sundler F. Occurrence and neonatal development of gastrin immunoreactivity in the digestive tract of the rat. Cell Tissue Res. 1974;149(2):275–281. doi: 10.1007/BF00222279. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F. Characterization of antral gastrin cells with region-specific antisera. J Histochem Cytochem. 1977 Dec;25(12):1317–1321. doi: 10.1177/25.12.925341. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Rubin B., Engel S. L., Pluscec J., Sheehan J. T. Cholecystokinin-pancreozymin: recent developments. Am J Dig Dis. 1970 Feb;15(2):149–156. doi: 10.1007/BF02235646. [DOI] [PubMed] [Google Scholar]
- Preiser H., Schmitz J., Maestracci D., Crane R. K. Modification of an assay for trypsin and its application for the estimation of enteropeptidase. Clin Chim Acta. 1975 Mar 10;59(2):169–175. doi: 10.1016/0009-8981(75)90025-x. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Rehfeld J. F. Radioimmunoassay in diagnosis, localization and treatment of endocrine tumours in gut and pancreas. Scand J Gastroenterol Suppl. 1979;53:33–38. [PubMed] [Google Scholar]
- Ryan J. P., Ryave S. Efffect of vasoactive intestinal polypeptide on gallbladder smooth muscle in vitro. Am J Physiol. 1978 Jan;234(1):E44–E46. doi: 10.1152/ajpendo.1978.234.1.E44. [DOI] [PubMed] [Google Scholar]
- Ryan J., Cohen S. Interaction of gastrin I, secretin, and cholecystokinin on gallbladder smooth muscle. Am J Physiol. 1976 Mar;230(3):553–556. doi: 10.1152/ajplegacy.1976.230.3.553. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Strunz U., Domschke W., Mitznegg P., Domschke S., Schubert E., Wünsch E., Jaeger E., Demling L. Analysis of the motor effects of 13-norleucine motilin on the rabbit, guinea pig, rat, and human alimentary tract in vitro. Gastroenterology. 1975 Jun;68(6):1485–1491. [PubMed] [Google Scholar]
- Vagne M., Grossman M. I. Cholecystokinetic potency of gastrointestinal hormones and related peptides. Am J Physiol. 1968 Oct;215(4):881–884. doi: 10.1152/ajplegacy.1968.215.4.881. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Wong H. C., Dockray G. J. Bombesin-like peptides in mammals. Fed Proc. 1979 Aug;38(9):2315–2319. [PubMed] [Google Scholar]
- Zetler G. Antagonism of cholecystokinin-like peptides by opioid peptides, morphine or tetrodotoxin. Eur J Pharmacol. 1979 Nov 23;60(1):67–77. doi: 10.1016/0014-2999(79)90053-0. [DOI] [PubMed] [Google Scholar]
- Zetler G., Cannon D., Powell D., Skrabanek P., Vanderhaeghen J. J. Crude substance P from brain contains a cholecystokinin-like peptide. Naunyn Schmiedebergs Arch Pharmacol. 1978 Nov;305(2):189–190. doi: 10.1007/BF00508292. [DOI] [PubMed] [Google Scholar]


