Abstract
1. The background activity was observed in γ and α efferent fibres and in group I and II fibres innervating the muscle gastrocnemius lateralis or medialis. The reflex effects of ipsilateral and contralateral sural nerve stimulations on the muscle efferents were analysed together with their consequences upon the afferents of the same muscle. The observations were made in the decerebrated cat without opening the neural loops between the muscle and the spinal cord.
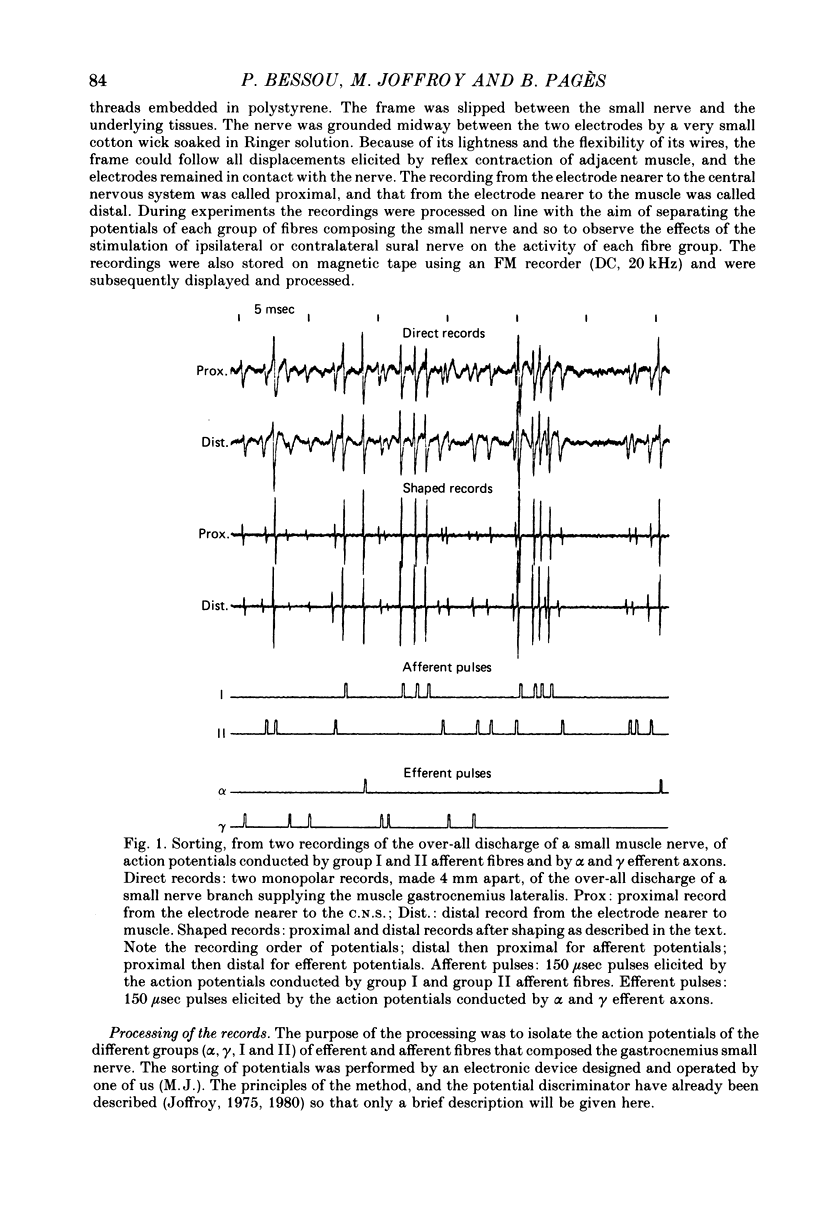
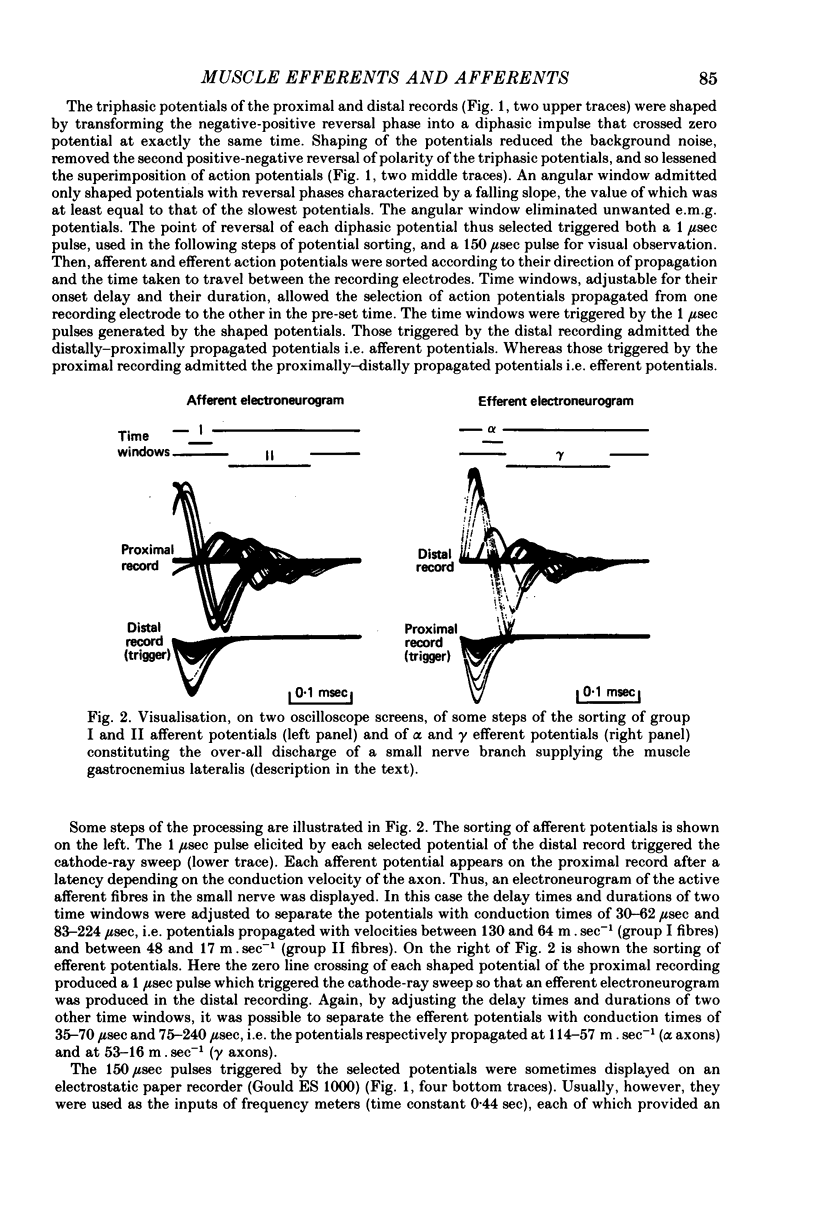
2. The multi-unit discharges of each category of fibres were obtained, on line, by an original electronic device (Joffroy, 1975, 1980) that sorted the action potentials from the whole electrical activity of a small branch of gastrocnemius lateralis or medialis nerve according to the direction and velocity of propagation of the potentials.
3. The small nerve may be regarded as a representative sample of different functional groups of fibres conducting faster than 12 m.sec-1 and supplying gastrocnemius muscles.
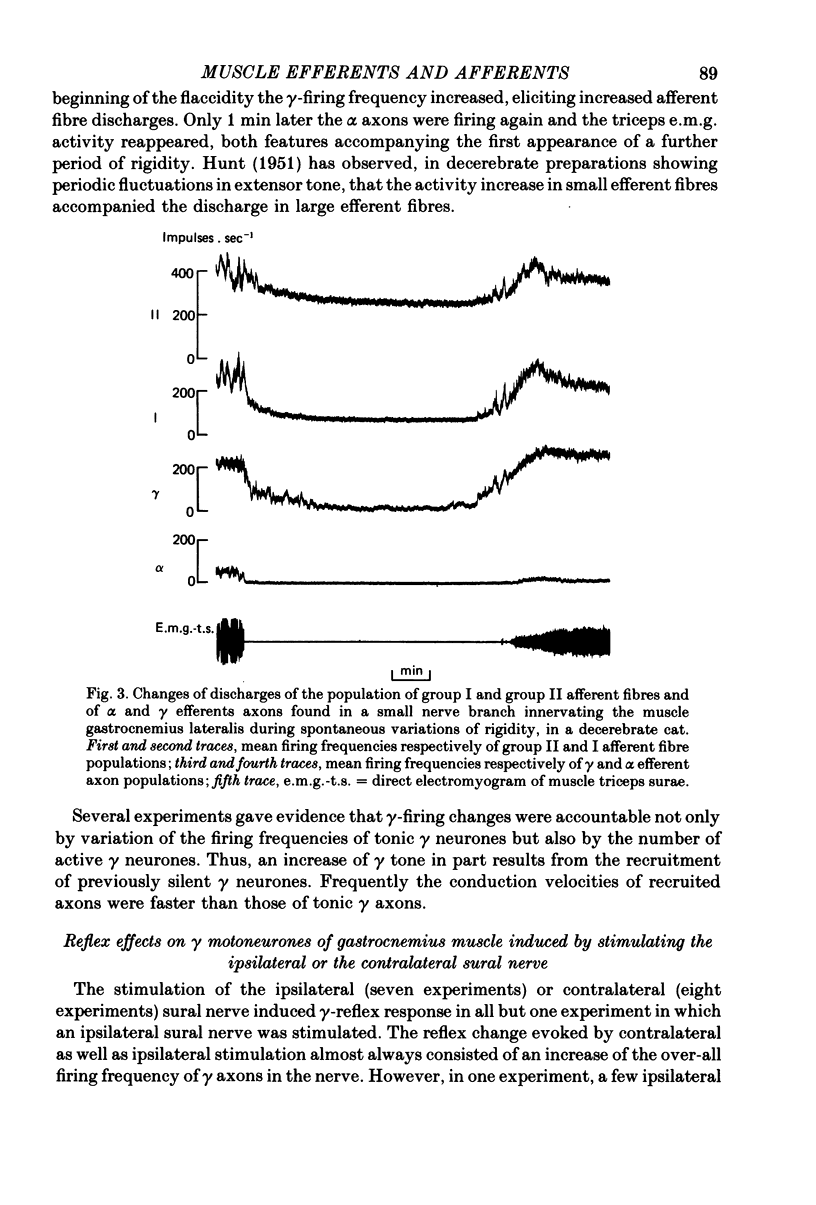
4. Some γ efferents were always tonically firing except when a transient flaccid state developed. Usually the α efferents were silent, probably because the muscle was fixed close to the minimal physiological length.
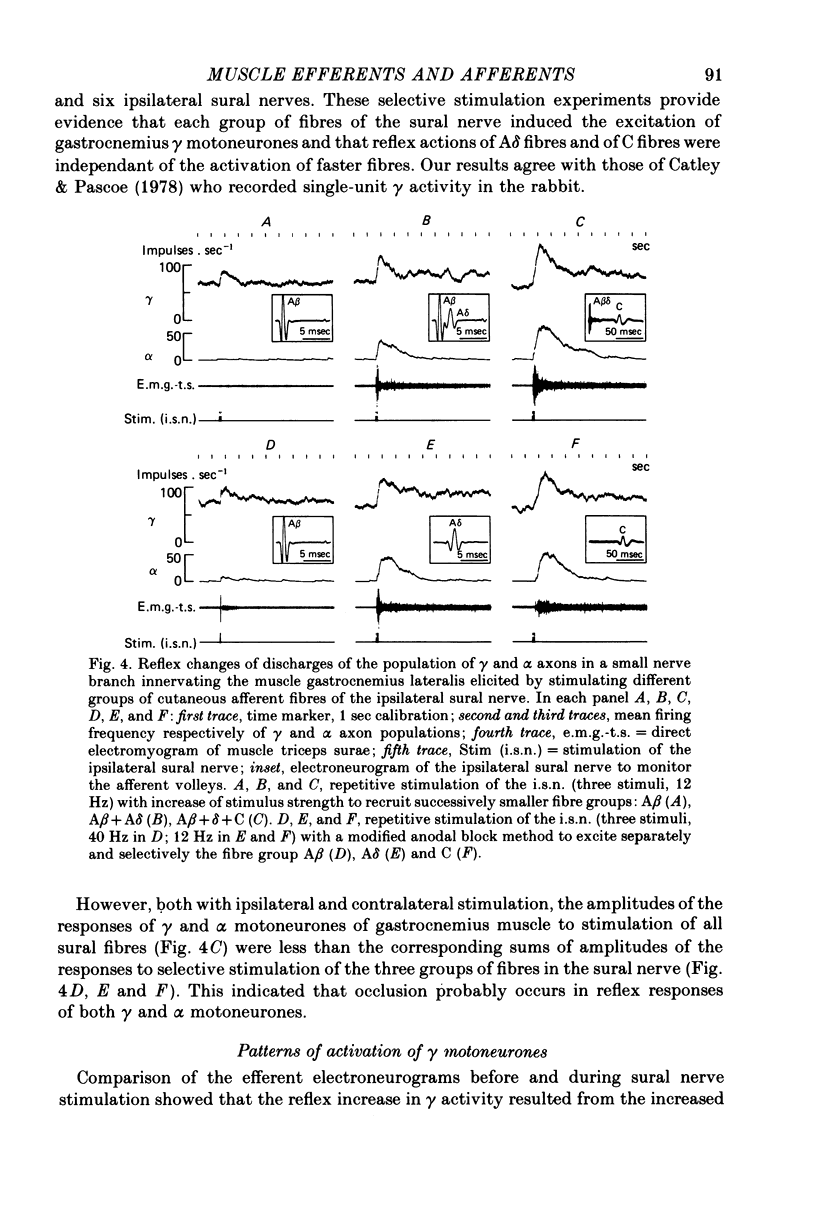
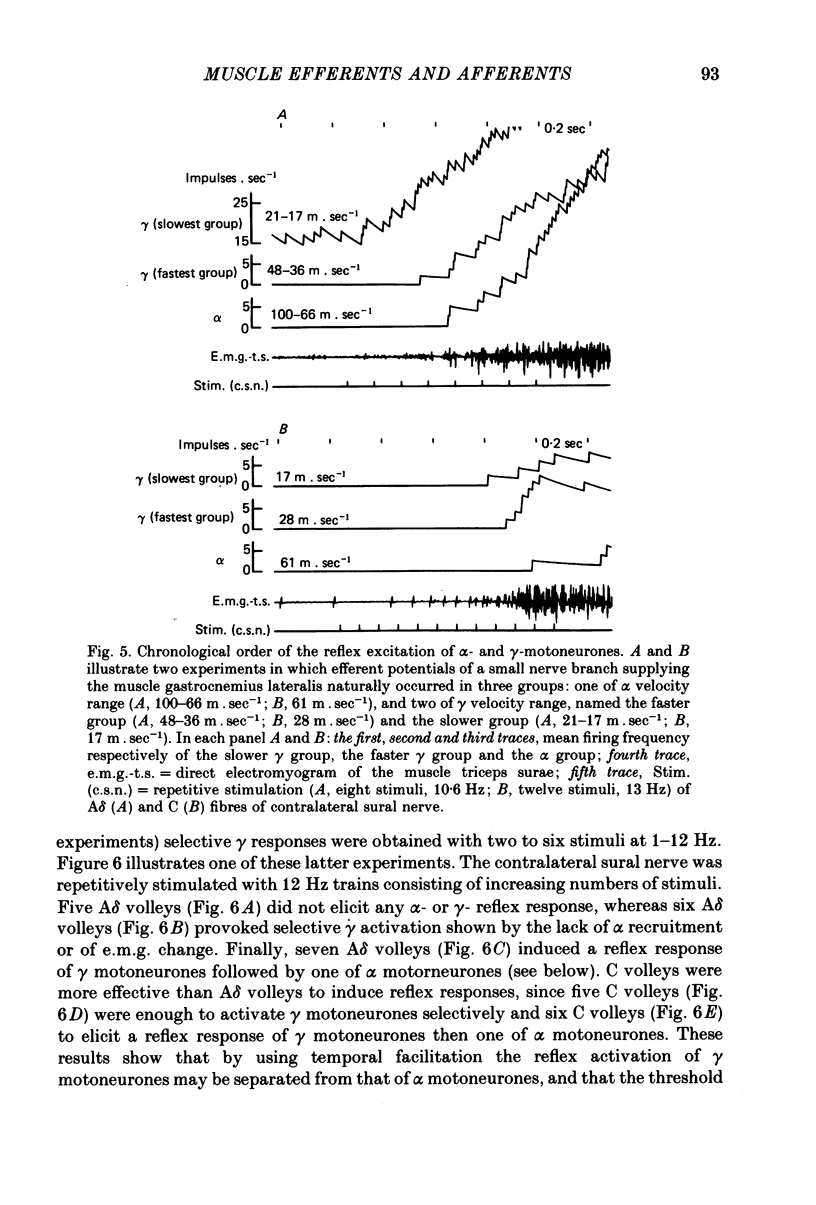
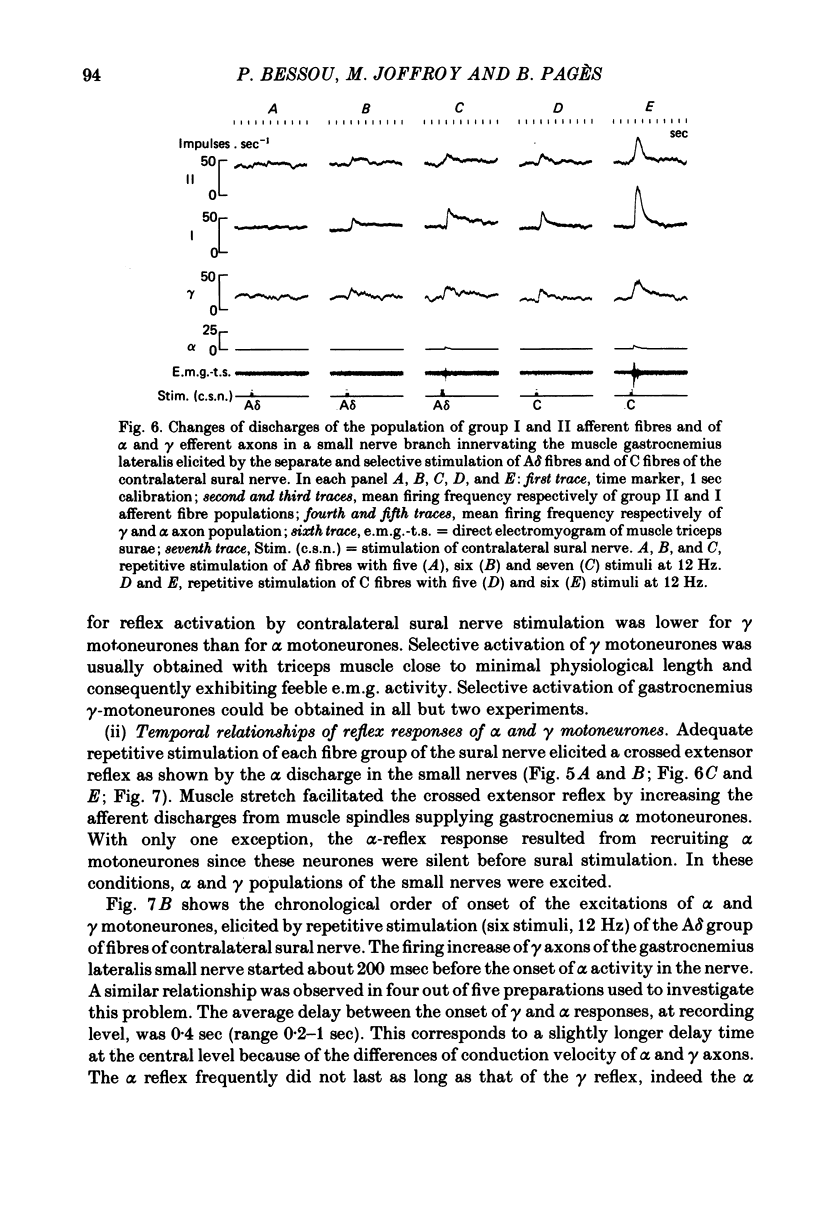
5. Separate and selective stimulations of Aβ, Aδ and C fibres of ipsilateral and contralateral sural nerve showed that each group could induce the excitation of γ neurones. The reciprocal inhibition period of α efferents during a flexor reflex was only once accompanied by a small decrease in γ-firing.
6. The reflex increase of over-all frequency of γ efferents resulted from an increased firing rate of tonic γ neurones and from the recruitment of γ neurones previously silent. When the γ efferents in the small nerve naturally occurred in two subgroups, the slower-conducting subgroup (mainly composed of tonic γ axons) was activated before the faster-conducting subgroup (mostly composed by γ axons with no background discharge). Some rare exceptions were found, however.
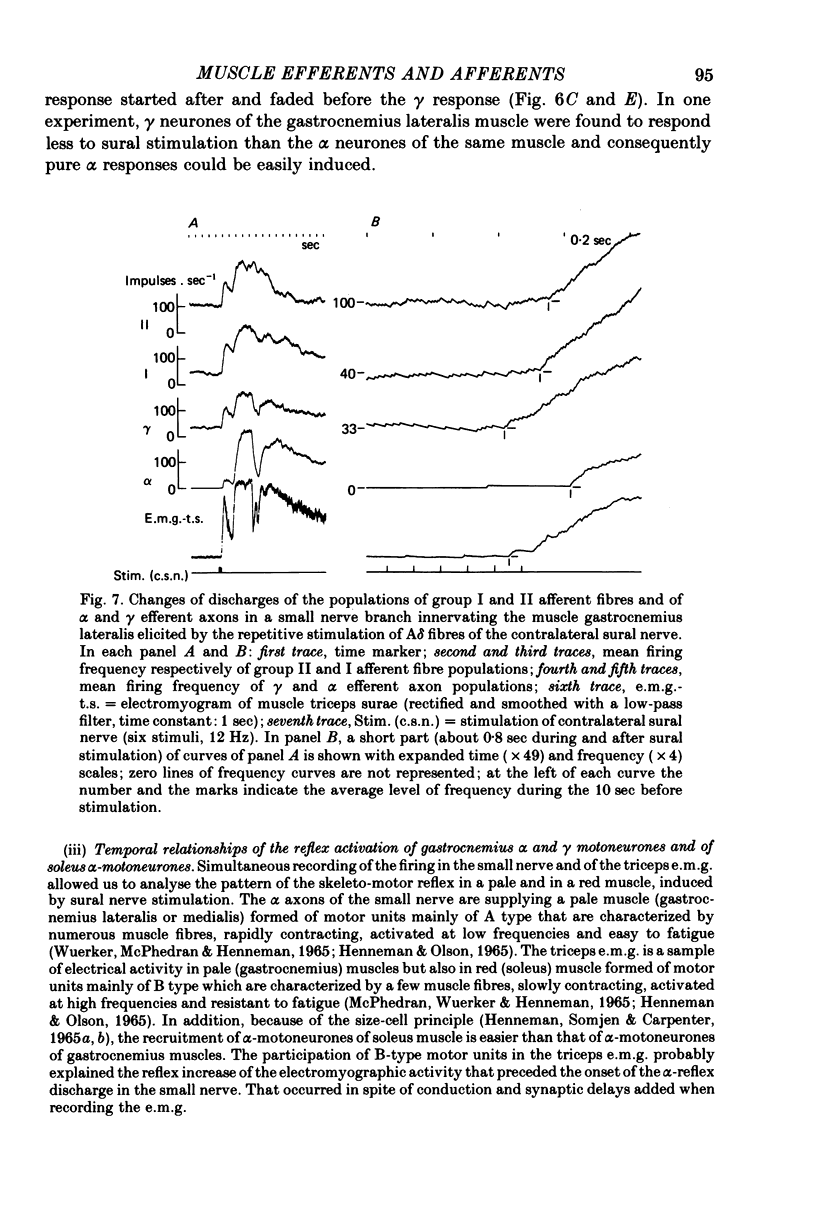
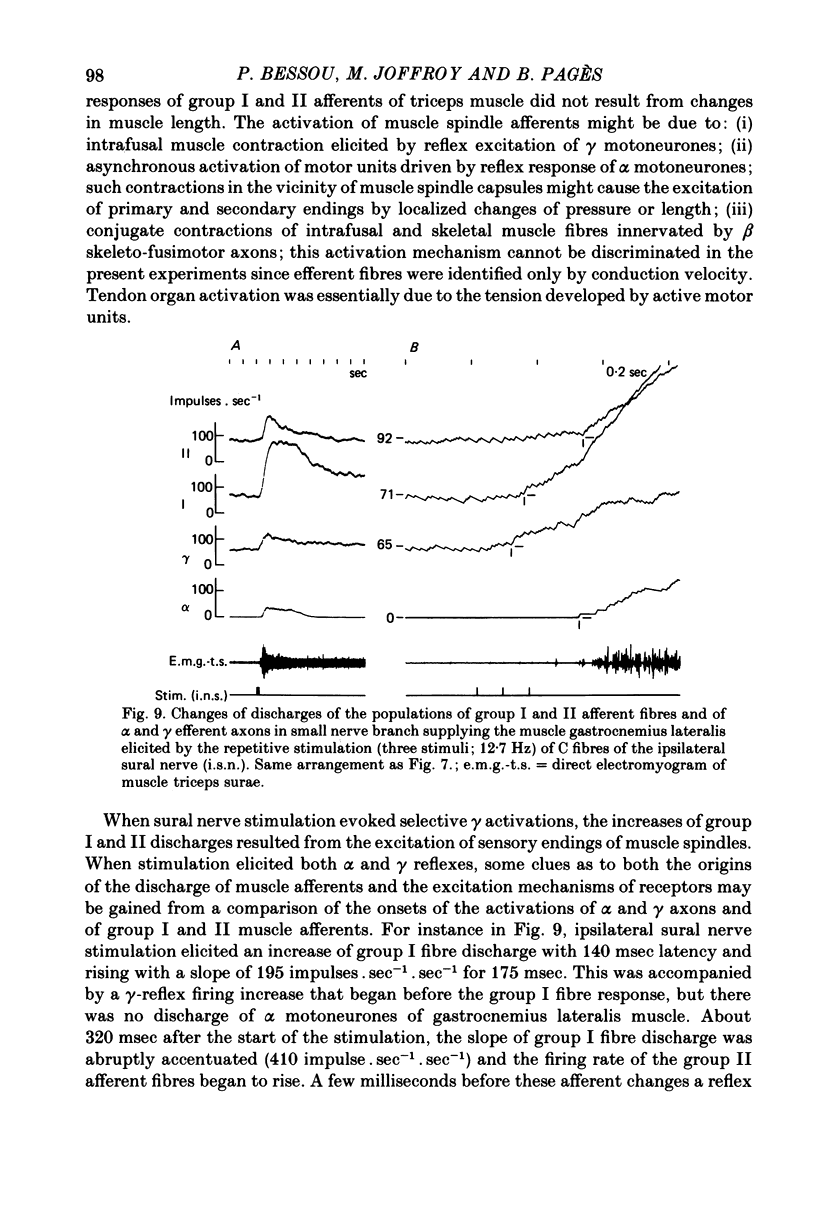
7. The selective activation of γ efferents could be obtained with short-and low-frequency stimulation. When, with stronger stimulations, γ—α co-activation was observed, the onset of the γ-firing increase preceded by 100-600 msec that of the α discharge in the small nerve. Likewise, the onset of the γ-efferent response preceded the increase of over-all electromyographic activity of the whole triceps muscle but only by 10-100 msec. The discrepancy could be due to the soleus α motoneurones being activated earlier than the α-motoneurones of gastrocnemius muscle, according to the size principle. In only one experiment, the α-firing onset preceded the γ-firing increase.
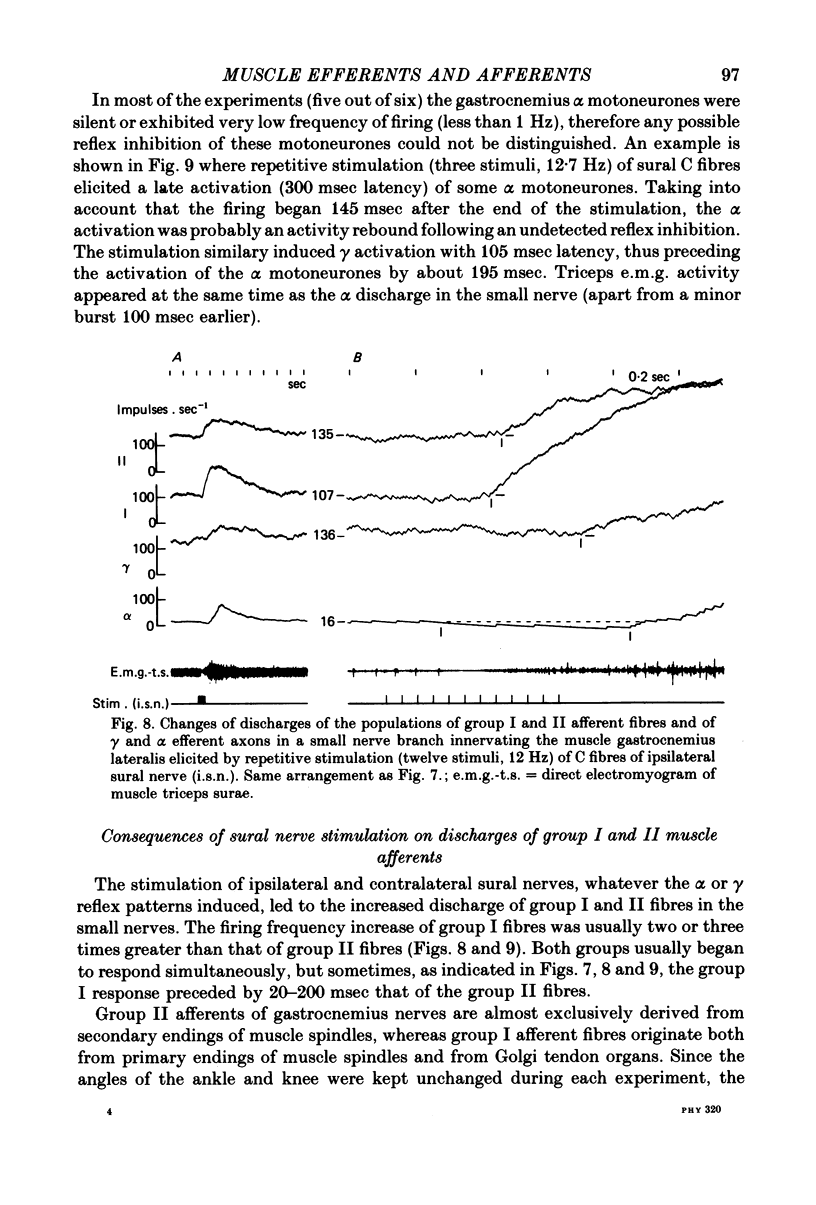
8. Stimulations of ipsilateral or contralateral nerve, whatever the α or γ reflex patterns, always led to increased firing rates of group I and II fibres of the small nerve. The origins of the discharge of group I and II muscle afferents and the excitation mechanisms of the receptors involved are considered. Some aspects of the mechanism of the reflex control of movement are discussed in the light of these results.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accornero N., Bini G., Lenzi G. L., Manfredi M. Selective Activation of peripheral nerve fibre groups of different diameter by triangular shaped stimulus pulses. J Physiol. 1977 Dec;273(3):539–560. doi: 10.1113/jphysiol.1977.sp012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Johansson H., Kalistratov G. The influence of group II muscle afferents and low threshold skin afferents on dynamic fusimotor neurones to the triceps surae of the cat. Brain Res. 1977 Aug 19;132(1):153–158. doi: 10.1016/0006-8993(77)90713-2. [DOI] [PubMed] [Google Scholar]
- Banks R. W., Bessou P., Joffroy M., Pagès B. Reflex responses of pools of gastrocnemius lateralis gamma motoneurones elicited by ipsilateral sural nerve stimulation in the cat [proceedings]. J Physiol. 1979 Nov;296:107P–107P. [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stauffer E. K., Stuart D. G. Correlation analysis of muscle spindle responses to single motor unit contractions. J Physiol. 1976 May;257(2):325–336. doi: 10.1113/jphysiol.1976.sp011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D. M., Pascoe J. E. The reflex effects of sural nerve stimulation upon gastrocnemius fusimotor neurones of the rabbit [proceedings]. J Physiol. 1978 Mar;276:32P–32P. [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., LUNDBERG A. Electrophysiological studies on gamma motoneurones. Acta Physiol Scand. 1960 Sep 30;50:32–40. doi: 10.1111/j.1748-1716.1960.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. An application of cumulative sum technique (cusums) to neurophysiology [proceedings]. J Physiol. 1977 Feb;265(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KAADA B. R. Influence of stimulation of central nervous structures on muscle spindles in cat. Acta Physiol Scand. 1952;27(2-3):130–160. doi: 10.1111/j.1748-1716.1953.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Grillner S., Hongo T., Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiol Scand. 1969 Apr;75(4):592–613. doi: 10.1111/j.1748-1716.1969.tb04414.x. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., PAINTAL A. S. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958 Sep 23;143(2):195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951 Dec 28;115(4):456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Vallbo A. B. Single unit recordings from muscle nerves in human subjects. Acta Physiol Scand. 1969 Jul;76(3):321–334. doi: 10.1111/j.1748-1716.1969.tb04475.x. [DOI] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Joffroy M. Méthode de discrimination des potentiels unitaires constituant l'activité complexe d'un filet nerveux non sectionné. J Physiol (Paris) 1975 Jul;70(2):239–252. [PubMed] [Google Scholar]
- KOBAYASHI Y., OSHIMA K., TASAKI I. Analysis of afferent and efferent systems in the muscle nerve of the toad and cat. J Physiol. 1952 Jun;117(2):152–171. doi: 10.1113/jphysiol.1952.sp004737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C. The mammalian small-nerve fibers: a system for efferent nervous regulation of muscle spindle discharge. Res Publ Assoc Res Nerv Ment Dis. 1952;30:24–47. [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HETEROGENEOUS PALE MUSCLE (M. GASTROCNEMIUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:85–99. doi: 10.1152/jn.1965.28.1.85. [DOI] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HOMOGENEOUS RED MUSCLE (SOLEUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:71–84. doi: 10.1152/jn.1965.28.1.71. [DOI] [PubMed] [Google Scholar]
- MERTON P. A. The silent period in a muscle of the human hand. J Physiol. 1951 Jun;114(1-2):183–198. doi: 10.1113/jphysiol.1951.sp004610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K. S. Vertebrate fusimotor neurones and their influences on motor behavior. Prog Neurobiol. 1978;11(3-4):249–307. doi: 10.1016/0301-0082(78)90015-1. [DOI] [PubMed] [Google Scholar]
- VOORHOEVE P. E., van KANTEN R. Reflex behaviour of fusimotor neurones of the cat upon electrical stimulation of various afferent fibers. Acta Physiol Pharmacol Neerl. 1962;10:391–407. [PubMed] [Google Scholar]


