Abstract
Abnormally fast transcription elongation can lead to detrimental consequences such as transcription–replication collisions, altered alternative splicing patterns and genome instability. Therefore, elongating RNA polymerase II (Pol II) requires mechanisms to slow its progression, yet the molecular basis of transcription braking remains unclear. RECQL5 is a DNA helicase that functions as a general elongation factor by slowing down Pol II. Here we report cryo-electron microscopy structures of human RECQL5 bound to multiple transcription elongation complexes. Combined with biochemical analysis, we identify an α-helix of RECQL5 responsible for binding Pol II and slowdown of transcription elongation. We further reveal that the transcription-coupled DNA repair (TCR) complex allows Pol II to overcome RECQL5-induced transcription braking through concerted actions of its translocase activity and competition with RECQL5 for engaging Pol II. Additionally, RECQL5 inhibits TCR-mediated Pol II ubiquitination to prevent activation of the DNA repair pathway. Our results suggest a model in which RECQL5 and the TCR complex coordinately regulate transcription elongation rates to ensure transcription efficiency while maintaining genome stability.
Subject terms: Cryoelectron microscopy, Transcription, RNA
Using cryo-electron microscopy and biochemistry, Zhang et al. reveal that the DNA helicase RECQL5 and the transcription-coupled DNA repair complex coordinate to regulate transcription elongation rates and maintain genome stability.
Main
During eukaryotic transcription, release from the promoter-proximal pausing region is mediated by the positive transcription elongation factor b (P-TEFb) that allows the formation of an activated elongation complex (EC*) to commence productive elongation1,2. EC* allows RNA polymerase II (Pol II) to drastically increase its speed and processivity and contains additionally the elongation factors DSIF (a two-subunit complex of SPT4 and SPT5), SPT6 and PAF (composed of CTR9, PAF1, SKI8, LEO1 and CDC73)2. However, the Pol II elongation rate is highly dynamic in cells and varies within individual genes and between different genes3,4. The transcription elongation rate affects co-transcriptional processes such as splicing, termination, mRNA modification and genome stability3–11. While a slow elongation rate was observed over exons, exon–intron junctions and poly(A) sites10–14, the mechanism underlying this transcription braking during productive elongation remains unclear.
RECQL5 was identified as a general elongation factor that controls transcription elongation rate15. Previous studies showed that RECQL5 decreases the Pol II elongation speed in vivo, inhibits transcription initiation and elongation in vitro and is crucial for maintaining genome stability15–19. Unlike other members of the RecQ helicase family, RECQL5 is only present in higher eukaryotes and uniquely interacts with Pol II18,20. Mice deficient in RECQL5 have increased risks of developing cancers, with profound sister chromatid exchange and double-stranded DNA breaks21,22. Additionally, RECQL5 plays a critical role at the intersection of transcription, replication and DNA recombination22–28.
Here, we report cryo-electron microscopy (cryo-EM) structures of human RECQL5 bound to multiple transcription elongation complexes. Together with biochemical analysis, we define the molecular basis of RECQL5-induced transcription slowdown. Furthermore, we identify that the transcription-coupled DNA repair (TCR) complex reactivates Pol II following RECQL5-induced transcription braking and restores the elongation rate. Our results provide a mechanistic understanding of the interplay between RECQL5 and the TCR complex in regulating Pol II elongation rate, thereby maintaining genome stability.
Results
Structure of the RECQL5 transcription complexes
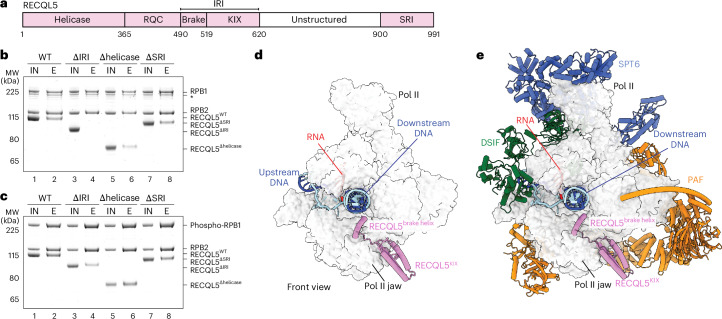
All RecQ helicases contain a core helicase domain followed by the RecQ C-terminal domain. RECQL5 has a unique C-terminal extension that consists of two protein-interacting domains, an internal Pol II-interacting (IRI) domain and a SET2–RPB1-interacting (SRI) domain (Fig. 1a). The IRI domain contains the kinase-inducible domain-interacting (KIX) domain. While the SRI domain was suggested to interact with the flexible phosphorylated Pol II C-terminal domain (CTD)29–31, a medium-resolution cryo-EM reconstruction revealed that the KIX domain may bind to the Pol II jaw17.
Fig. 1. Structures of the EC–RECQL5 and EC*–RECQL5 complexes.
a, Domain organization of RECQL5. Brake, brake helix; RQC, RecQ C-terminal domain. b,c, Pulldown of non-phosphorylated Pol II (b) and phosphorylated Pol II (c) with different RECQL5 constructs using the Twin-Strep-tag on Pol II. Asterisk indicates RPB1 with CTD degradation. IN, input; E, elution; MW, molecular weight; WT, wild type. All pulldowns were repeated in triplicate. d, Structure of the EC–RECQL5 complex in front view. Pol II is shown in light gray surface representation, with RECQL5 shown in pink cartoon. Template DNA is colored dark blue, non-template DNA is in cyan, and RNA is in red. e, Structure of the EC*–RECQL5 complex with the elongation factors SPT6 (blue), DSIF (green) and PAF (orange) in cartoon representation.
To gain insights into the interactions between Pol II and RECQL5, we performed pulldowns using a Twin-Strep-tagged human Pol II and RECQL5 constructs with individual domains (helicase, IRI or SRI) deleted (Fig. 1a–c). In the absence of Pol II phosphorylation, deletion of the IRI domain eliminated RECQL5 binding to Pol II (Fig. 1b, lane 4), indicating that the IRI domain engages the Pol II body and does not require Pol II phosphorylation for binding, consistent with previous studies16,17,32. Deletion of either the helicase domain or the SRI domain had no effect on RECQL5 binding to non-phosphorylated Pol II.
During transcription elongation, the Pol II CTD is hyperphosphorylated33,34. Compared to non-phosphorylated Pol II, we observed a slight increase in RECQL5 binding to in vitro phosphorylated Pol II (Fig. 1c, lane 2). This increase is contributed to by the interaction between the RECQL5 SRI domain and the phosphorylated Pol II CTD (Extended Data Fig. 1a), which enhances the affinity of RECQL5 to Pol II through multiple interaction sites. Deletion of the IRI domain strongly reduced RECQL5 binding to phosphorylated Pol II, whereas loss of the SRI domain resulted in a minor decrease of bound RECQL5 (Fig. 1c, compare lanes 4–8). Overall, these results demonstrate that the IRI domain confers the major contribution for RECQL5 interactions with Pol II, whereas the SRI domain enhances binding to phosphorylated Pol II.
Extended Data Fig. 1. Preparation of the EC-RECQL5, EC*-RECQL5 and EC-TCR-RECQL5 complexes.
a, Pulldown of the SRI domain of RECQL5 with MBP-tagged Pol II CTD. Phosphorylation of the CTD (pCTD) is required for its interaction with the SRI domain. This pulldown was repeated in duplicates. b, Assembled EC-RECQL5 (left), EC*-RECQL5 (middle), and EC-TCR-RECQL5 (right) complexes on 4-12% NuPAGE Bis-Tris gels run in MOPS, stained with Instant Blue. The complex assembly experiments were repeated in triplicates. c, Schematic of the DNA-RNA scaffolds used for structural and biochemical analysis. d, Representative micrographs of EC-RECQL5 (left), EC*-RECQL5 (middle) and EC-TCR-RECQL5 (right) complexes collected on the 300 kV Titan Krios with a Falcon4i detector in electron counting mode. A total of 8,602 micrographs were collected for the EC-RECQL5 complex, 60,808 micrographs for the EC*-RECQL5 complex and 11,156 micrographs for the EC-TCR-RECQL5 complex. e, Two-dimensional averages of the EC-RECQL5 (left), EC*-RECQL5 (middle) and EC-TCR-RECQL5 (right) complexes.
To define the molecular basis of the interactions between RECQL5 and Pol II, we determined cryo-EM structures of RECQL5 bound to both a transcription elongating Pol II (EC) and the activated transcription elongation complex EC* (Fig. 1d,e and Supplementary Video 1). To reconstitute the EC–RECQL5 and EC*–RECQL5 complexes, recombinantly purified human RECQL5 was incubated with preassembled phosphorylated transcription elongation complexes and subjected to single-particle cryo-EM analysis (Extended Data Figs. 1–5 and Table 1). The cryo-EM reconstructions reached an overall resolution of 2.8 Å for the EC–RECQL5 complex and 2.0 Å for the EC*–RECQL5 complex. We observed cryo-EM densities corresponding to the RECQL5 IRI domain in both reconstructions. Modeling of the EC and EC* structures2,35 and fitting of AlphaFold predictions of RECQL5 and elongation factors resulted in models of the EC–RECQL5 and EC*–RECQL5 complexes with good stereochemistry (Fig. 1d,e and Table 1).
Extended Data Fig. 5. Cryo-EM densities of the EC*-RECQL5 and EC-RECQL5 reconstructions.
a, Cryo-EM densities of the non-sharpened overall map showing the RECQL5-Pol II interface and closeup view of the sharpened overall map showing the interface between the RECQL5 brake helix and the RPB1 jaw domain. The views and colours are the same as in Fig. 2a. b, Close-up view of RECQL5 brake helix (pink) protruding towards the downstream DNA (blue and cyan) in the sharpened EC-RECQL5 map. Cα atoms of R496 and R502 residues are shown in magenta spheres. c-e, Cryo-EM densities of the non-sharpened focused refined maps with subtracted particles for SPT6Core-Pol IIStalk (c), SPT6Core (d) and PAF (e). f, Sharpened cryo-EM densities showing the SPT6tSH2-CDC73 interaction. g, Sharpened cryo-EM densities at the Pol II active centre reveal the post-translocated state that can accept incoming nucleotide. h, i, Model versus map FSC for the overall map of EC-RECQL5 (h) and EC*-RECQL5 (i) using the FSC standard of 0.5. The cryo-EM densities were coloured according to Fig. 1e.
Table 1.
Statistics of cryo-EM reconstructions and structural models
| EC*–RECQL5 | EC–RECQL5 | EC–TCR–RECQL5 | |||
|---|---|---|---|---|---|
| Overall (EMD-52443) |
SPT6 stalk (EMD-52441) |
PAF (EMD-52442) |
Overall (EMD-52440) |
Overall (EMD-52449) |
|
| Data collection and processing | |||||
| Microscope | Titan Krios | Titan Krios | Titan Krios | ||
| Voltage (kV) | 300 | 300 | 300 | ||
| Camera | Falcon 4i | Falcon 4i | Falcon 4i | ||
| Magnification | ×96,000 | ×96,000 | ×96,000 | ||
| Pixel size (Å/pixel) | 0.8156 | 0.815 | 0.8156 | ||
| Electron exposure (e−/Å2) | 39.9 | 42.7 | 36.6 | ||
| Exposure rate (e−/Å2/frame) | 0.997 | 1.06 | 0.915 | ||
| Number of frames per movie | 40 | 40 | 40 | ||
| Defocus range (μm) | 0.5–2.0 | 0.5–2.0 | 0.5–2.0 | ||
| Automation software | EPU | EPU | EPU | ||
| Symmetry imposed | C1 | C1 | C1 | ||
| Initial particle numbers | 1,183,806 | 564,329 | 96,839 | ||
| Final particle numbers | 314,016 | 66,771 | 19,458 | ||
| Map sharpening B factor (Å2) | −20 | −68.1 | −67 | −60 | −40 |
| Map resolution (Å, FSC = 0.143) | 2.0 | 2.5 | 2.5 | 2.8 | 3.5 |
| Refinement | |||||
| Initial models used (PDB) | 6TED, 7B0Y, AlphaFold models | 7B0Y, AlphaFold | 9HVQ, 8B3D | ||
| Model resolution (Å) | 2.1 | 3.05 | 3.8 | ||
| Model composition | |||||
| Non-hydrogen atoms | 63,421 | 33,905 | 51,212 | ||
| Protein residues | 7,652 | 4,014 | 6,202 | ||
| Nucleic acid residues | 86 | 86 | 88 | ||
| Ligands | Zn:8 Mg:1 | Zn:8 Mg:1 | Zn:10 Mg:2 | ||
| B factors (Å2) | |||||
| Protein | 174.65 | 27.26 | 240.99 | ||
| Nucleotide | 161.77 | 87.11 | 162.41 | ||
| Ligand | 67.63 | 81.41 | 204.51 | ||
| R.m.s. deviations | |||||
| Bond lengths (Å) | 0.006 | 0.004 | 0.009 | ||
| Bond angles (°) | 0.613 | 0.497 | 0.850 | ||
| Validation | |||||
| MolProbity score | 1.47 | 1.45 | 1.81 | ||
| Clashscore | 5.82 | 4.64 | 7.02 | ||
| Poor rotamers (%) | 0.75 | 0 | 1.65 | ||
| Cß deviations (%) | 0 | 0 | 0 | ||
| Ramachandran plot | |||||
| Favored (%) | 97.19 | 96.63 | 96.15 | ||
| Allowed (%) | 2.75 | 3.37 | 3.83 | ||
| Outliers (%) | 0.07 | 0.0 | 0.02 | ||
| PDB code | 9HVQ | 9HVO | 9HWG | ||
FSC, Fourier shell correlation; PDB, Protein Data Bank.
The RECQL5 brake helix is crucial for Pol II binding
The structures reveal that RECQL5 binds directly to the jaw domain of the Pol II RPB1 subunit via its KIX domain (Figs. 1d,e and 2a–c and Supplementary Video 1). Additionally, an α-helix (residues 490–519) N terminal to the KIX domain rests on the jaw domain of RPB1 and extends toward the minor groove of the downstream DNA. This N-terminal α-helix is resolved at ~3 Å, showing densities of side chains contacting Pol II, while the KIX domain is more mobile with a local resolution of 4–4.5 Å (Extended Data Fig. 5a). Basic residues at the N terminus of the helix, R496 and R502, are located near the DNA phosphate backbone (Extended Data Fig. 5b). We named this α-helix of RECQL5 the brake helix. Therefore, the IRI domain of RECQL5 comprises the brake helix and the KIX domain (Fig. 1a). Strikingly, deleting the brake helix completely abolished RECQL5 binding to non-phosphorylated Pol II, underlining the importance of the brake helix in the Pol II–RECQL5 interaction (Fig. 2d).
Fig. 2. The RECQL5 brake helix is crucial for Pol II binding and transcription slowdown.
a, Close-up view of interactions between RECQL5 (pink) and the jaw domain of the Pol II RPB1 subunit (light gray). The brake helix protrudes toward the downstream DNA (blue and cyan). b, Detailed views of interactions between the RECQL5 brake helix and the RPB1 jaw domain. c, The electrostatic surface potential of the RPB1 jaw domain (left) and the RECQL5 KIX domain (right) in book view with a range of ±5 kT/e, where blue and red represent positively and negatively charged areas, respectively. The dashed lines highlight the interface areas between RPB1 and RECQL5 that are oppositely charged. d, Pulldown of non-phosphorylated Pol II with wild-type and brake helix-deleted RECQL5. e,f, RNA extension assays of EC* in the presence of various RECQL5 constructs (Δbrake helix, ΔIRI, Δhelicase, ΔSRI, IRI, helicase, SRI (e); RECQL5D157A, Δhelicase (f)) showing that the brake helix and the helicase activity of RECQL5 are critical for slowing down transcription elongation. nt, nucleotides. g, Pulldown of EC and EC* with RECQL5 using the Twin-Strep-tag on Pol II. All assays and pulldowns were repeated in triplicate.
The brake helix of RECQL5 forms extensive contacts with the jaw domain of RPB1 (Fig. 2a,b). Bulky side chains of W504, F507, Y508 and M512 on the brake helix engage a hydrophobic surface of RPB1 that is formed by F1239, L1243, I1261, F1284 and I1288 (Fig. 2b and Extended Data Fig. 5a). The brake helix interaction with the RPB1 jaw is further stabilized by multiple hydrogen bonds, including R515 of the brake helix with D1281 and M1279 of RPB1, Q511 of the brake helix with RPB1 M1279, and Y508 of the brake helix with RPB1 F1239 (Fig. 2b and Extended Data Fig. 5a).
The interface between the RECQL5 KIX domain and RPB1 shows charge complementarity (Fig. 2c). A negatively charged surface of RPB1 formed by glutamates and aspartates docks onto a positively charged patch on helices 1 and 3 of the KIX domain (Fig. 2a,c).
Residues at the interface of RECQL5 and RPB1 are highly conserved among higher eukaryotes (Extended Data Fig. 6a,b), indicating that RECQL5 may likewise interact with Pol II in other eukaryotic species. Cross-linking-coupled mass spectrometry data identified cross-links between the RECQL5 IRI domain and the Pol II jaw, further validating the structural data (Extended Data Fig. 7a–c and Extended Data Table 1).
Extended Data Fig. 6. Sequence alignments and superposition of the EC-RECQL5 structure with transcription elongation complexes.
a, Sequence alignment of the RECQL5 IRI domain, with the position of the brake helix and the three α-helices of the KIX domain shown as cylinders. Residues of the brake helix in contact with RPB1 are marked with pink stars. b, Sequence alignment of the RPB1 jaw domain. Residues that contact the RECQL5 brake helix are marked with grey stars. c, Electromobility shift assay (EMSA) of wildtype and D157A mutant RECQL5. D157A mutation does not affect RECQL5 binding to the DNA. The assay was repeated in triplicate. d, Superposition of the EC*-RECQL5 complex with EC*-nucleosome (PDB: 8A3Y)36 on Pol II showed no clash between RECQL5 and the downstream nucleosome. EC* and RECQL5 are depicted as described in Fig. 1e and histones are shown in white. e, Superposition of the EC-RECQL5 complex with EC-TFIIS (PDB: 8A40)37 on Pol II showed that the RECQL5 KIX domain and TFIIS share overlapping binding site on the Pol II jaw. TFIIS is shown in light blue.
Extended Data Fig. 7. Crosslinking-coupled mass spectrometry analysis of the EC*-RECQL5 complex.
a, Crosslinking network of the EC*-RECQL5 complex with BS3. Crosslinks of RECQL5 (pink) with Pol II (grey) and elongation factors SPT6 (blue), DSIF (green) and PAF (orange) are shown. Interlinks were detected between RECQL5 and RPB1, RPB2, RPB5, PAF components, and the N-termini of SPT4, SPT5 and SPT6. b, Crosslinks mapped onto the EC*-RECQL5 structure. Crosslinks among all subunits of the EC*-RECQL5 complex within the permitted distance of 30 Å are shown. c, Histogram of the number of crosslinks and distances between crosslinked Cα pairs mapped onto the EC*-RECQL5 structure. 69% of mapped crosslinks fall within the permitted crosslinking distance of 30 Å (blue). Crosslinks outside the permitted distance (red) are likely due to complex aggregation and flexibility. d, Pulldown of RECQL5 with elongation factors (EF) SPT6, DSIF, PAF and RTF1 using an MBP tag on RECQL5. SPT6 formed a stoichiometric complex with RECQL5, while DSIF and PAF bound to RECQL5 at reduced levels. RTF1, a loosely associated factor of PAF, showed no interaction with RECQL5. IN: input, E: elution. Pulldown was repeated in triplicates.
Extended Data Table 1.
Crosslinking mass-spectrometry of the EC*-RECQL5 complex
RECQL5 induces transcription braking
Previous studies reported that RECQL5 inhibits transcription initiation and elongation in vitro in the presence of initiation factors16,17. Both the IRI and helicase domains were found to be important for the inhibitory effect of RECQL5, but helicase activity was not required16. To understand the role of RECQL5 in transcription elongation, we recapitulated productive transcription elongation using the EC* complex containing elongation factors DSIF, SPT6 and PAF (Fig. 2e,f).
RECQL5 slows down transcription elongation effectively, resulting in accumulation of shorter transcripts and Pol II stalling at different locations compared to EC* transcription in the absence of RECQL5 (Fig. 2e, compare lane 2 to lane 1). Deletion of either the brake helix alone or the entire IRI domain partially relieved RECQL5-induced transcription braking (Fig. 2e, lanes 3 and 4), indicating that the brake helix plays a critical role in slowing down Pol II. The brake helix extends toward the minor groove of the downstream DNA, possibly providing a steric hindrance for Pol II to proceed with transcription (Fig. 2a and Extended Data Fig. 5b). Interestingly, deletion of the helicase domain not only partially restored transcription but also recovered the Pol II stalling pattern to that of EC* (Fig. 2e, compare lane 5 to lane 1). On the other hand, although Pol II is phosphorylated in EC*, loss of the SRI domain did not impact the inhibitory effect of RECQL5 on transcription (Fig. 2e, lane 6). The IRI domain alone moderately slowed down transcription elongation, whereas neither the helicase domain nor the SRI domain in isolation affected transcription (Fig. 2e, lanes 7–9).
As we could not observe cryo-EM density corresponding to the RECQL5 helicase domain, it may induce transcription braking either as a roadblock for Pol II movement by binding to the downstream DNA or as a helicase to partially unwind the downstream DNA. To investigate whether the helicase activity of RECQL5 is required for its inhibitory effect on productive transcription elongation, we used RECQL5D157A, a previously characterized helicase mutant16. The electromobility shift assay showed that the D157A mutation does not affect RECQL5 binding to DNA (Extended Data Fig. 6c). Strikingly, the RECQL5D157A mutant failed to slow down transcription to the same extent as wild-type RECQL5 (Fig. 2f). Instead, both the Pol II stalling pattern and the level of transcription braking by RECQL5D157A resemble those of RECQL5Δhelicase (Fig. 2f, compare lanes 3 and 4). These data reveal that the helicase activity of RECQL5 is important for slowing down productive transcription elongation, which differs from previous transcription assays using initiation factors. RECQL5 helicase activity may contribute to the change in Pol II stalling pattern by partially unwinding and distorting the downstream DNA. Overall, our results show that, while the brake helix is essential for RECQL5 binding to Pol II, both the brake helix and the helicase activity of RECQL5 are required to slow down transcription during productive elongation.
RECQL5 binding accommodates elongation factors
Our structures reveal that RECQL5 engages both EC and EC* identically on the Pol II jaw via its IRI domain and does not change the binding modes of elongation factors DSIF, SPT6 and PAF on Pol II, which are required for productive elongation (Fig. 1d,e). Additionally, we find that the presence of elongation factors does not impair RECQL5 binding to EC (Fig. 2g). Furthermore, RECQL5 forms a complex with the elongation factor SPT6 in our pulldown assay and interacts weakly with DSIF and PAF (Extended Data Fig. 7d). Supporting this, our cross-linking-coupled mass spectrometry data showed interlinks of RECQL5 with elongation factors SPT6, DSIF and PAF (Extended Data Fig. 7a–c and Extended Data Table 1). Interlinks were found between the RECQL5 helicase domain and the N-terminal regions of SPT4, SPT5 and SPT6, implying possible interactions of the RECQL5 helicase domain with elongation factors that may contribute to helicase domain-mediated transcriptional braking during elongation. These results reveal that RECQL5 binding is compatible with EC* and that the elongation factors may be involved in the recruitment of RECQL5 to Pol II.
Although RECQL5 causes transcription elongation to proceed at a lower speed, it does not arrest Pol II to a complete stop. The active site of Pol II adopts a post-translocated state that can accept an incoming nucleotide substrate (Extended Data Fig. 5g). In addition, superposition of the EC*–RECQL5 structure with a structure of EC* transcribing into a nucleosome36 revealed that RECQL5 binding at the Pol II jaw has no clash with the downstream nucleosome (Extended Data Fig. 6d).
RECQL5 and UVSSA have overlapping binding sites on Pol II
The binding site of the RECQL5 KIX domain on the Pol II jaw overlaps with that of transcription factor IIS (TFIIS)37 (Extended Data Fig. 6e) and UV-stimulated scaffold protein A (UVSSA)38, a component of the TCR complex (Fig. 3a,b and Extended Data Fig. 8a). TFIIS helps Pol II to overcome stalling by stimulating cleavage of backtracked RNA, allowing reactivation of transcription elongation37. Previous studies found that RECQL5 inhibited TFIIS-mediated transcription readthrough of elongation blocks17 and UVSSA prevented TFIIS-mediated cleavage of backtracked RNA38. Nevertheless, the functional relationship between RECQL5 and UVSSA remains unclear.
Fig. 3. RECQL5 and the TCR complex have overlapping binding sites on Pol II.
a, Domain organization of UVSSA. b, Superposition of the EC–RECQL5 structure with the EC–TCR structure (PDB 8B3D)38 revealed that the RECQL5 brake helix (pink) and the C-terminal hinged helix of UVSSA (dark purple) bind to the same site on the Pol II jaw (gray), while the RECQL5 KIX domain and the UVSSA zinc finger domain occupy the same binding site. The two helices are positioned next to the K1268 loop, which is the main target of Pol II ubiquitination. c, RECQL5 and the TCR complex can bind to Pol II simultaneously. The competition assay was performed using the Twin-Strep-tag on non-phosphorylated Pol II, with either RECQL5 (lane 6) or the TCR complex (lane 7) added to Pol II first and repeated in triplicate. MBP, maltose-binding protein. d, Cryo-EM structure of the EC–TCR–RECQL5 complex. Pol II is shown in light gray surface representation, while RECQL5 (pink) and the TCR components CSB (teal), CSA (yellow), DDB1 (dark red), ELOF1 (lime) and UVSSA (dark purple) are shown as cartoon. The RECQL5 IRI domain binds to the Pol II jaw and prevents stable engagement of the UVSSA C-terminal region.
Extended Data Fig. 8. RECQL5 and UVSSA compete for the same binding site on Pol II.
a, Superposition of the EC-RECQL5 structure with the EC-TCR structure (PDB: 8B3D)38 reveals that the brake helix of RECQL5 (pink) and the C-terminal hinged helix of UVSSA (dark purple) bind to the same site on the Pol II jaw, while the RECQL5 KIX domain and the UVSSA zinc finger domain occupy the same binding site. The TCR complex engages the Pol II additionally with CSB and ELOF1. EC-TCR complex and RECQL5 are depicted as in Fig. 3d. Right panel: The RECQL5 brake helix (pink) and the UVSSA hinged helix (dark purple) have opposite directionality. b, Competition assay of RECQL5ΔSRI and the TCR complex on phosphorylated Pol II, with either RECQL5ΔSRI (lane 2) or the TCR complex (lane 4) added to phosphorylated Pol II first. This assay was repeated in triplicates. c, CSB ubiquitination by CRL4 is not affected by the presence of either wildtype or ΔIRI mutant RECQL5. This assay was repeated in triplicates.
Aside from UVSSA, the TCR complex contains the Cockayne syndrome proteins, CSB and CSA, and the DNA damage binding protein 1 (DDB1). Transcription elongation factor 1 homolog (ELOF1) helps to stably position UVSSA on the Pol II jaw, allowing targeted Pol II ubiquitination and activation of the DNA repair pathway38,39. The TCR complex interacts with Pol II via three contact points mediated by CSB, UVSSA and ELOF1 (ref. 38) (Extended Data Fig. 8a). CSB competes with DSIF for the binding site on the Pol II stalk, thereby displacing DSIF from Pol II38.
A comparison of our EC–RECQL5 structure and the EC–TCR structure38 revealed that not only the RECQL5 KIX domain and the UVSSA zinc finger domain bind to the same site on the Pol II jaw, but the RECQL5 brake helix also aligns perfectly onto the C-terminal hinged helix of UVSSA, extending into the minor groove of the downstream DNA (Fig. 3a,b and Extended Data Fig. 8a). The RECQL5 brake helix and the UVSSA C-terminal helix have opposite directionality (Extended Data Fig. 8a, right). While both helices use an arginine (UVSSA, R669; RECQL5, R515) at the end of the helix to form hydrogen bonds with the Pol II jaw, RECQL5 engages the hydrophobic patch of the Pol II jaw with bulky aromatic residues, which are replaced by an isoleucine and a valine in UVSSA. The overlapping binding sites of RECQL5 and UVSSA on Pol II, combined with the ability of CSB to promote Pol II forward translocation on non-damaged templates40–42, prompted us to investigate the role of the TCR complex in restoring RECQL5-induced transcription slowdown.
Structure of the EC–TCR–RECQL5 complex
We first tested whether the TCR complex can displace RECQL5 from Pol II using competition assays, in which either RECQL5 or the TCR complex was pre-incubated with EC first to form a complex before addition of the competitor (Fig. 3c and Extended Data Fig. 8b). The competition assays were performed either in the absence of Pol II phosphorylation or using the RECQL5ΔSRI mutant to eliminate the SRI–CTD interaction. This restricts RECQL5 association with Pol II to the IRI–jaw interaction, which overlaps with the UVSSA binding site. In the competition assay using non-phosphorylated EC, the amount of RECQL5 and TCR factors bound to Pol II remained the same across all conditions, regardless of the order in which RECQL5 and the TCR complex were added (Fig. 3c). The same result was obtained using phosphorylated EC; both RECQL5ΔSRI and the TCR complex bound simultaneously to Pol II regardless of their order of addition (Extended Data Fig. 8b). Despite occupying the same binding site on the Pol II jaw, RECQL5 binding to Pol II is not diminished by the TCR complex, likely due to the RECQL5 IRI domain being a stronger binder. Both the TCR complex and RECQL5 can coexist on the Pol II surface as the TCR complex engages Pol II additionally via CSB and ELOF1 (Extended Data Fig. 8a).
To further investigate whether the TCR complex and RECQL5 can bind to Pol II simultaneously and which protein occupies the Pol II jaw, we determined the cryo-EM structure of an EC–TCR–RECQL5 complex with an overall resolution of 3.5 Å (Fig. 3d, Extended Data Fig. 9, Supplementary Video 1 and Table 1). The TCR complex engages Pol II in the same mode as the EC–TCR structure38, with CSB interacting with the upstream DNA and Pol II clamp, while ELOF1 binds stably to the RPB2 lobe near the DNA entry tunnel. RECQL5 binds to the Pol II jaw identically as in the EC–RECQL5 structure, with the brake helix extending toward the downstream DNA. On the other hand, only weak cryo-EM densities corresponding to the UVSSA N-terminal VHS domain were observed (Extended Data Fig. 9). The structure reveals that both the TCR complex and RECQL5 can bind to Pol II at the same time, although RECQL5 prevents the stable engagement of the UVSSA C-terminal region to the Pol II jaw.
Extended Data Fig. 9. Cryo-EM data processing of the EC-TCR-RECQL5 complex.
a-f, After removing bad particles with 2D-classification, the remaining particles were CTF-refined (a, b). Focused 3D-classification without alignment were performed using a soft mask on RECQL5 (c). Particles containing densities of RECQL5 were combined and 3D-refined (d). Following focused 3D-classification on TCR were performed to remove the EC-RECQL5 only particles (e), resulting in the final EC-TCR-RECQL5 reconstruction at 3.5 Å resolution (f). g, FSC curves of the final map for the EC-TCR-RECQL5 complex. h, Model versus map FSC for the overall map of EC-TCR-RECQL5 using the FSC standard of 0.5. The cryo-EM densities were coloured according to Fig. 3d.
The TCR complex reactivates transcription elongation
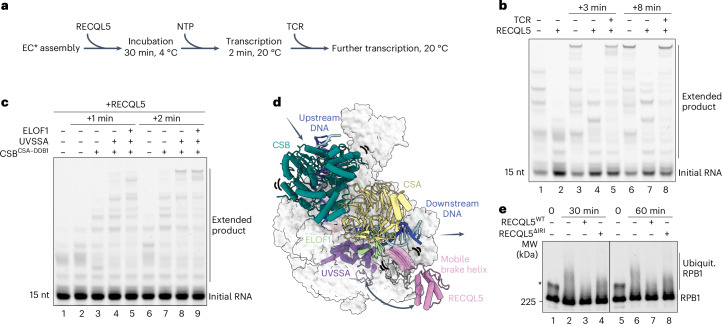
We next investigated whether the TCR complex can restore transcription elongation following RECQL5-induced transcription braking in vitro. In this assay, we allowed RECQL5-mediated transcription slowdown to progress for 2 min before adding the TCR components to proceed with further transcription (Fig. 4a–c). In the absence of TCR factors, elongation is slow due to transcription braking by RECQL5. Addition of CSB–CSA–DDB1 partially relieved the transcription slowdown, whereas addition of the entire TCR complex including UVSSA and ELOF1 completely restored transcription to the same level as EC* in the absence of RECQL5 (Fig. 4b,c).
Fig. 4. The TCR complex reactivates transcription elongation.
a, Schematic of the EC* RNA extension assay in the presence of RECQL5 and the TCR complex. NTP, nucleoside triphosphate. b, The TCR complex reactivates elongation following RECQL5-induced transcription braking. Initial transcription proceeded for 2 min without or with RECQL5 (lanes 1 and 2) before addition of the TCR complex and further transcription for 3 or 8 min. c, Both CSB and UVSSA are important to restore transcription elongation. Initial transcription with RECQL5 proceeded for 2 min before addition of TCR factors and further transcription for 1 or 2 min. d, Proposed mechanism on how the TCR complex allows Pol II to overcome RECQL5-induced transcription slowdown. The concerted action of CSB translocase activity and the competition by UVSSA on the Pol II jaw makes the RECQL5 brake helix mobile, allowing reactivation of transcription elongation. UVSSA is depicted in transparent cartoon at its approximate location to illustrate the potential competition with RECQL5. DDB1 is hidden to provide a clear view of the brake helix. e, RECQL5 inhibits TCR-mediated Pol II ubiquitination shown on the western blot using an RPB1 antibody. Asterisk indicates endogenously phosphorylated RPB1 at the 0-min time point. Ubiquit., ubiquitinated. All assays were repeated in triplicate.
Reactivation of transcription elongation is mediated by the translocase activity of CSB, which binds to the upstream DNA and hydrolyzes ATP to promote forward movement of Pol II during active transcription40,43. This disrupts the interaction between the downstream DNA and the RECQL5 brake helix and partially restores the elongation rate. Additionally, the UVSSA C-terminal hinged helix, stabilized by ELOF1, competes for the same binding site as the RECQL5 brake helix on the Pol II jaw. Destabilized RECQL5 brake helix by CSB translocase activity allows UVSSA to displace the RECQL5 brake helix, thereby relieving RECQL5-induced transcription slowdown (Fig. 4d).
RECQL5 inhibits TCR-mediated Pol II ubiquitination
When Pol II encounters DNA damage, recruitment of the TCR complex triggers Pol II ubiquitination and activation of the DNA repair pathway44,45. K1268 located in a loop of the jaw domain of the RPB1 subunit is the main target for Pol II ubiquitination, serving as a master switch to regulate transcription and DNA repair46,47. Correct positioning of the UVSSA C-terminal hinged helix next to the K1268 loop is required for accurate Pol II ubiquitination38. Our structures reveal that the brake helix of RECQL5 competes with the UVSSA C-terminal hinged helix for the same binding site next to the K1268 loop (Fig. 3b). We therefore hypothesized that, while the TCR complex restores the transcription elongation speed, RECQL5 may prevent Pol II ubiquitination to avoid excessive triggering of the DNA repair pathway in the absence of DNA damage.
To investigate the effect of RECQL5 on TCR-mediated Pol II ubiquitination, we performed in vitro Pol II ubiquitination assays using the E3 ligase CRL4 (Fig. 4e and Extended Data Fig. 8c). The E3 ligase CRL4 targets both Pol II and CSB for ubiquitination in response to UV damage, with Pol II ubiquitination leading to activation of the DNA repair pathway46–48. Remarkably, RECQL5 specifically inhibits RPB1 ubiquitination without affecting CSB ubiquitination, demonstrating the additional role of RECQL5 in preventing excessive Pol II ubiquitination during transcription elongation in the absence of DNA damage. Deleting the IRI domain strongly reduced the inhibitory effect of RECQL5 (Fig. 4e), supporting the idea that RECQL5 prevents Pol II ubiquitination by competing with UVSSA for the same binding site on the Pol II jaw.
Discussion
It is increasingly evident that transcription elongation is far more complex than initially thought, with transcription elongation serving as a key regulatory step in gene expression. Uncontrolled Pol II elongation speed disrupts co-transcriptional processes and compromises genome stability, with mutations affecting Pol II speed found to be embryonic lethal in mice3–11,49. Furthermore, transcription speed has been shown to adapt in response to internal and external stimuli50–52. Nevertheless, the mechanism by which Pol II decelerates and re-accelerates during productive elongation remains poorly understood.
In this study, we define the molecular basis of transcription braking by the elongation factor RECQL5 and reactivation of elongation by the TCR complex (Fig. 5 and Supplementary Video 1). Our structural and biochemical data suggest a model in which RECQL5 induces transcription braking by engaging the fast-moving EC* during productive elongation (Fig. 5a). RECQL5 slows down transcription via its brake helix that protrudes toward the downstream DNA and its helicase activity that may partially unwind the downstream DNA (Fig. 5b,c). Slowly moving Pol II triggers the recruitment of the TCR complex to probe for possible DNA damage (Fig. 5d). In the absence of DNA damage or if the obstacle can be bypassed, the TCR complex reactivates transcription elongation through a joint effort of CSB and UVSSA. While CSB uses its translocase activity to push Pol II forward, UVSSA competes with RECQL5 for the same binding site, thereby displacing RECQL5 from the Pol II jaw. At the same time, RECQL5 inhibits specifically TCR-mediated Pol II ubiquitination, but not that of CSB, thereby preventing excess activation of the DNA repair pathway (Fig. 5e). CSB polyubiquitination followed by degradation by the proteasome allows the release of the TCR complex from Pol II and the reestablishment of EC* (ref. 48) (Fig. 5f). Although not critical in our in vitro experiments, the interaction of RECQL5 with the phosphorylated Pol II CTD greatly increases the local concentration of RECQL5 and may allow swift rebinding of RECQL5 to the Pol II body.
Fig. 5. RECQL5 and the TCR complex coordinate to regulate transcription elongation speed.
a,b, RECQL5 recruitment to productive elongating EC* (a) slows down transcription through the brake helix and its helicase activity (b). c, The brake helix resides next to the loop harboring the residue K1268, which is the main target of Pol II ubiquitination. d, Reactivation of transcription elongation requires the joint effort of CSB and UVSSA in the TCR complex, displacing RECQL5 from the Pol II jaw. e, At the same time, RECQL5 inhibits TCR-mediated Pol II ubiquitination to prevent activation of the DNA repair pathway. f, CSB ubiquitination results in displacement of the TCR complex and restoration of EC*.
Supporting this model, loss of RECQL5 leads to an increased transcription elongation rate and transcription-induced genome instability in vivo15, demonstrating the role of RECQL5 as a general elongation factor. In the absence of RECQL5, sites with elevated transcription elongation were enriched in double-stranded DNA breaks, which appears to be associated with replication15,53,54. Therefore, RECQL5 may slow down transcription elongation to avoid transcription–replication collisions. On the other hand, reduced transcription elongation was observed in Cockayne syndrome group B cells55 and CSB stimulated the transcription elongation rate in vitro40,41,56, supporting a role of CSB in activating transcription elongation. Furthermore, CSB was isolated from whole-cell extracts as a part of a large complex that contains Pol II and was involved in regulating nucleosome positioning42,57.
Concurrent to our work, a complementary preprint by Ariza et al.58 revealed the structure of the RECQL5 helicase domain that binds to the downstream DNA and contacts Pol II, supporting our hypothesis that the helicase domain acts on the downstream DNA. The RECQL5 helicase domain was proposed to function as a roadblock to inhibit transcription58, likely based on previous studies showing that RECQL5 helicase activity was not required for the inhibition of transcription initiation16,17. However, as RECQL5 controls transcription elongation rather than initiation in cells15, we found that RECQL5 helicase activity is crucial for inducing transcription braking during productive elongation. Therefore, it is possible that the RECQL5 helicase domain uses its helicase activity to partially unwind the downstream DNA and simultaneously functions as a roadblock to slow down transcription. A later preprint by Sebesta et al.59 showed that the R502A mutation in the brake helix impaired transcription repression by RECQL5. R502 is located near the phosphate backbone of the downstream DNA in our reconstruction (Extended Data Fig. 5b). This supports our hypothesis that the interaction between positively charged residues on the brake helix and the downstream DNA creates steric hindrance for Pol II to proceed with transcription.
In sum, our data suggest that RECQL5 acts as a brake to slow down transcription elongation, while the TCR complex functions as an accelerator to overcome transcription braking. The coordinated actions of RECQL5 and the TCR complex ensure efficient transcription elongation while maintaining genome stability and possibly preventing transcription–replication collision and allowing co-transcriptional events to occur. Our work provides a framework for future studies on regulation of the transcription elongation rate and its regulatory roles in gene expression.
Methods
Cloning and protein expression
Sequences for human RECQL5, CSA, CSB, UVSSA, DDB1, CUL4A, RBX1, ELOF1 and UbcH5b were amplified from human cDNA. The sequence for human Pol II CTD was amplified from the codon-optimized RPB1 sequence (GenScript). Sequences for RECQL5, Pol II CTD, CSB and CUL4A were cloned into the 438C vector (Addgene, 55220) with an N-terminal His6-MBP tag; those for CSA and RBX1 were cloned into the 438A vector with no tag (Addgene, 55218); those for DDB1 and UVSSA were cloned into the 438B vector (Addgene, 55219) with an N-terminal His6 tag, and those for ELOF1 and UbcH5b were cloned into the 1C vector (Addgene, 29654) with an N-terminal His6-MBP tag using ligation-independent cloning60. Sequences for CSA and DDB1 as well as CUL4A and RBX1 were further combined into single vectors.
The following primers were used for cloning: RECQL5_Fw, TACTTCCAATCCAATGCAATGAGCAGCCACCATACCACC; RECQL5_Rv, TTATCCACTTCCAATGTTATTATCATCTCTGGGGGCCACAC; CSA_Fw, TACTTCCAATCCAATCGATGCTGGGGTTTTTGTCCGCAC; CSA_Rv, TTATCCACTTCCAATGTTATTATCATCCTTCTTCATCACTGCTGCTCC; CSB_Fw, TACTTCCAATCCAATGCAATGCCAAATGAGGGAATCCCCCA; CSB_Rv, TTATCCACTTCCAATGTTATTATTAGCAGTATTCTGGCTTGAGTTTCCAAATTCC; DDB1_Fw, TACTTCCAATCCAATGCAATGTCGTACAACTACGTGGTAACGG; DDB1_Rv, TTATCCACTTCCAATGTTATTACTAATGGATCCGAGTTAGCTCCTCCACA; UVSSA_Fw, TACTTCCAATCCAATGCAATGGATCAGAAACTTTCGAAGTTGGTAGAAGAG; UVSSA_Rv, TTATCCACTTCCAATGTTATTACTAGTTCAGTGCGTAGTTAAACTGGTTTGAAAAC; ELOF1_Fw, TACTTCCAATCCAATGCAATGGGGCGCAGAAAGTCAAAAC; ELOF1_Rv, TTATCCACTTCCAATGTTATTACTACTGATTGGCCGCCTCG; UbcH5b_Fw, TACTTCCAATCCAATGCAATGGCTCTGAAGAGAATCCACAAGGAAT; UbcH5b_Rv, TTATCCACTTCCAATGTTATTATTACATCGCATACTTCTGAGTCCATTCCC; CUL4A_Fw, TACTTCCAATCCAATGCAATGGCGGACGAGGCCC; CUL4A_Rv, TTATCCACTTCCAATGTTATTATCAGGCCACGTAGTGGTACTGATTC; RBX1_Fw, TACTTCCAATCCAATCGATGGCGGCAGCGATGGAT; RBX1_Rv, TTATCCACTTCCAATGTTATTACTAGTGCCCATACTTTTGGAATTCCCACT; Pol II-CTD_Fw, TACTTCCAATCCAATGCATACTCCCCAACCTCCCCTG; Pol II-CTD_Rv, TTATCCACTTCCAATGTTATTAGTTTTCCTCGTCGGAGTC.
Protein sequences cloned into the 438 vector series were expressed in High Five cells (Gibco, B85502). One liter of High Five cells in Sf-900 II SFM medium (Gibco) were infected with P2 virus generated in SF9 cells (Gibco, 12659017) and grown for 50–72 h. Cells were collected by centrifugation at 1,000g for 18 min, frozen in liquid nitrogen and stored at −80 °C before protein purification. All mutants of RECQL5 were generated using QuikChange61 and were expressed and purified as the wild-type proteins.
Proteins cloned into the 1C vector were expressed in BL21 (DE3)-RIL cells (Agilent, 230245) in LB medium. Expression was induced with 1 mM IPTG when the cell density reached an OD of 0.6–0.8. UbcH5b was expressed overnight at 18 °C, whereas ELOF1 was expressed at 37 °C for 4 h before collection and storage at −80 °C.
Protein purification
For all RECQL5 constructs, cell pellets were resuspended in lysis buffer (50 mM Tris-HCl, pH 7.5, 500 mM NaCl, 30 mM imidazole, 5% glycerol, 1 mM dithiothreitol (DTT)) supplemented with 1 mM PMSF and EDTA-free protease inhibitor tablets (Roche). The resuspended cells were sonicated, clarified by centrifugation and loaded onto a 5-ml HisTrap HP column (Cytiva). The column was washed with lysis buffer and eluted with lysis buffer supplemented with 300 mM imidazole. The eluate was applied onto a home-packed amylose column (NEB), washed with lysis buffer and eluted with amylose elution buffer (50 mM Tris-HCl, pH 7.5, 300 mM NaCl, 50 mM maltose, 5% glycerol, 1 mM DTT). The His6-MBP tag was cleaved with TEV protease overnight and applied to a HisTrap HP column to remove TEV protease, undigested protein and His6-MBP tag. Flowthrough was collected and further purified with HiLoad 16/600 Superdex 200-pg or HiLoad 16/600 Superdex 75-pg columns (Cytiva) in RECQL5 size exclusion chromatography (SEC) buffer (20 mM HEPES-NaOH, pH 7.5, 200 mM NaCl and 1 mM DTT). The peak fractions were concentrated using Amicon Ultra Centrifugal Filters (Merck). For purification of His6-MBP-tagged wild-type RECQL5, the eluate from the amylose column was diluted four times with buffer A (50 mM Tris-HCl, pH 7.5, 5% glycerol, 1 mM DTT) and applied onto a HiTrap Heparin HP column (Cytiva). The protein was eluted with a gradient of buffer A with 1 M NaCl before being subject to a final size exclusion purification step on the HiLoad 16/600 Superdex 200-pg column (Cytiva) in RECQL5 SEC buffer. Peak fractions were concentrated, frozen in liquid nitrogen and stored at −80 °C.
The His6-MBP-tagged human Pol II CTD was purified the same way as RECQL5 with a HisTrap HP column (Cytiva) followed by an amylose column (NEB). Following amylose elution, protein was diluted to 100 mM NaCl with buffer A, applied to a HiTrap Q HP column and eluted with a gradient of buffer A with 1 M NaCl. Peak fractions were concentrated and treated with lambda phosphatase in the presence of 1 mM MnCl2 to remove insect cell-derived phosphorylation. The lambda phosphatase-treated CTD was applied onto a Superdex 200 increase 10/300 GL column (Cytiva), and peak fractions were concentrated, frozen in liquid nitrogen and stored at −80 °C.
The porcine Pol II was purified from the Sus scrofa domesticus thymus as described35. In brief, the thymus was homogenized in a blender followed by polyethylenimine precipitation and ion exchange chromatography using Macro-Prep High Q resin (Bio-Rad). The eluate was precipitated using ammonium sulfate followed by affinity purification with an 8WG16 antibody column and a further UNO Q1 column (Bio-Rad). The final porcine Pol II sample was stored in Pol II storage buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 10 μM ZnCl2, 2 mM DTT). Porcine Pol II has a 99.9% sequence identity to human Pol II.
The Twin-Strep-tagged human Pol II was purified from HEK293 cells as described62, using the Twin-Strep-tag at the N terminus of RPB1. Cells were lysed in 0 M buffer (50 mM Tris-HCl, pH 7.9, 5 mM MgCl2, 0.5 mM EDTA, 10% glycerol, 2 mM DTT, 1 mM Na2S2O5, 1 mM PMSF) supplemented with EDTA-free protease inhibitor tablets (Roche) using sonication. Subsequently, an equal volume of 0.6 M ammonium sulfate buffer (0 M buffer with 0.6 M ammonium sulfate) supplemented with EDTA-free protease inhibitor tablets (Roche) and 0.01 mg ml−1 DNase (Sigma-Aldrich) was added, and the cells were sonicated further before centrifugation. Supernatant was precipitated with 50% saturated ammonium sulfate for 1 h at 4 °C, redissolved at 0.5 M ammonium sulfate, applied onto the Strep-Tactin XT 4Flow column (IBA) and eluted with 0.18 M buffer (0 M buffer with 0.18 M ammonium sulfate) supplemented with 50 mM biotin. The eluate was further purified with a UNO Q1 column (Bio-Rad) and eluted with a gradient of 0.18 M to 0.5 M buffer. Fractions containing Pol II were concentrated and buffer exchanged to Pol II storage buffer.
Human P-TEFb, PAF, DSIF, RTF1 and SPT6 were purified as described2. P-TEFb was expressed in High Five cells and purified using GSTrap 4B columns (Cytiva), followed by TEV cleavage and further purification using GSTrap 4B columns to remove the GST tag before the final size exclusion chromatography using a HiLoad 16/600 Superdex 200-pg column (Cytiva) in protein storage buffer (20 mM HEPES-NaOH, pH 7.5, 300 mM NaCl, 10% glycerol, 1 mM DTT). PAF was expressed in High Five cells and purified with a HisTrap HP column (Cytiva) followed by a HiTrap Q HP column (Cytiva). The eluate was treated with lambda phosphatase at 1 mM MnCl2 to remove insect cell-derived phosphorylation. Following TEV cleavage, the protein was reapplied onto the HisTrap HP column and further purified using a HiLoad 16/600 Superdex 200-pg column (Cytiva) in protein storage buffer. RTF1 and SPT6 were expressed in insect cells and purified with a HisTrap HP column followed by a home-packed amylose column (NEB). Following lambda phosphatase treatment and TEV cleavage, the proteins were reapplied onto the HisTrap HP column and further purified using the HiLoad 16/600 Superdex 200-pg column in protein storage buffer. DSIF was expressed in Escherichia coli and purified with a HisTrap HP column before TEV cleavage overnight. The protein was reapplied onto the HisTrap HP column followed by a HiTrap Q HP column and a final purification step with a HiLoad 16/600 Superdex 200-pg column in protein storage buffer.
Human TCR proteins were purified as described38. CSB was expressed in High Five cells and purified using a HisTrap HP column followed by a home-packed amylose column (NEB). The His6-MBP tag was cleaved with TEV protease overnight, and the protein was further purified with HiTrap Heparin HP and HiLoad 16/600 Superdex 200-pg columns in TCR storage buffer (20 mM HEPES-NaOH, pH 7.5, 400 mM NaCl, 5% glycerol, 1 mM DTT). UVSSA was expressed in High Five cells and purified using a HisTrap HP column before TEV cleavage overnight. The cleaved protein was applied onto a HisTrap HP column followed by a HiTrap Heparin HP column and a final purification step on a HiLoad 16/600 Superdex 200-pg column in TCR storage buffer. DDB1–CSA were coexpressed in High Five cells and purified with a HisTrap HP column and a HiTrap Q HP column before TEV cleavage overnight. The cleaved proteins were applied onto a HisTrap HP column and a HiTrap Heparin HP column before the final size exclusion step on the Superdex 200 Increase 10/300 GL column (Cytiva) in TCR storage buffer with 300 mM NaCl. ELOF1 was expressed in E. coli and purified with a HisTrap HP column and a home-packed amylose column before TEV cleavage overnight. The protein was reapplied onto the HisTrap HP column and further purified on a HiLoad 16/600 Superdex 75-pg column in protein storage buffer with 5% glycerol. CUL4A–RBX1 were coexpressed in High Five cells and purified using a HisTrap HP column followed by a home-packed amylose column (NEB). The His6-MBP tag was cleaved with TEV protease overnight, and the protein was further purified with a HisTrap HP column and a HiLoad 16/600 Superdex 200-pg column in TCR storage buffer with 300 mM NaCl. UbcH5b was purified as described for RECQL5, but the pH of the buffers was adjusted to 8.0, and the storage buffer was 20 mM HEPES-NaOH, pH 8.0, 100 mM NaCl, 1 mM DTT.
Typical yields of the purification are listed in the table below:
| Protein/complex | Typical yield (mg) | Normalized starting material |
|---|---|---|
| Sus scrofa Pol II | 2 | 1 kg thymus |
| Human Pol II | 0.1 | 1 l HEK293 |
| RECQL5 | 5 | 1 l High Five |
| PAF | 1.5 | 1 l High Five |
| SPT6 | 18 | 1 l High Five |
| DSIF | 1 | 1 l E. coli |
| RTF1 | 9 | 1 l High Five |
| P-TEFb | 0.4 | 1 l High Five |
| CSA–DDB1 | 0.5 | 1 l High Five |
| CSB | 1.6 | 1 l High Five |
| UVSSA | 0.2 | 1 l High Five |
| CUL4A–RBX1 | 13.5 | 1 l High Five |
| UbcH5b | 0.72 | 1 l E. coli |
| ELOF1 | 2.5 | 1 l E. coli |
| His6-MBP-CTD | 23 | 1 l High Five |
Sample preparation for cryo-EM
EC–RECQL5 and EC*–RECQL5 complexes were formed on a DNA–RNA scaffold with a mismatch bubble of 11 nucleotides. The same RNA and template DNA were used for both complexes: RNA, 5′-GAGAGGGAACCCACU-3′; template DNA, 5′-GCTCCCAGCTCCCTGCTGGCTCCGAGTGGGTTCTGCCGCTCTCAATGG-3′. For EC–RECQL5, a non-template DNA of the same length as the template DNA was used: 5′-CCATTGAGAGCGGCCCTTGTGTTCAGGAGCCAGCAGGGAGCTGGGAGC-3′. For EC*–RECQL5, a non-template DNA with a 15-nucleotide 3′ overhang was used: 5′-CCATTGAGAGCGGCCCTTGTGTTCAGGAGCCAGCAGGGAGCTGGGAGCCTTAGACAGCATGTC-3′. All oligonucleotides were synthesized by IDT and resuspended in water.
For the EC–RECQL5 complex, RNA was annealed with an equimolar amount of template DNA by incubating at 60 °C for 5 min, followed by a gradual decrease in temperature at a rate of 1 °C min−1 to a final temperature of 30 °C in 20 mM HEPES, pH 7.5, 100 mM NaCl and 3 mM MgCl2. Porcine Pol II (75 pmol) was incubated with the RNA–DNA hybrid (150 pmol) at 30 °C for 10 min, followed by addition of the non-template DNA (300 pmol) and incubation at 30 °C for another 10 min. The complex was phosphorylated with GSK3B (300 pmol) and ATP (1 mM) in SEC100 buffer (20 mM HEPES, pH 7.5, 100 mM NaCl, 3 mM MgCl2, 1 mM DTT) at 30 °C for 30 min, followed by incubation with RECQL5 (300 pmol) on ice for 30 min. The assembled EC–RECQL5 complex was applied to a Superdex 200 Increase 3.2/300 column (Cytiva) equilibrated in SEC150 buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 3 mM MgCl2 and 1 mM DTT). The peak fraction of EC–RECQL5 was used for freezing grids.
For the EC*–RECQL5 complex, the Pol II elongation complex was prepared essentially as described for the EC–RECQL5 complex. Final protein amounts used for complex formation were 100 pmol porcine Pol II, 200 pmol RNA–DNA hybrid and 400 pmol non-template DNA. The complex was mixed with elongation factors (SPT6, DSIF and PAF, 200 pmol each) and phosphorylated with P-TEFb (33 pmol) and ATP (1 mM) in SEC100 buffer for 30 min at 30 °C. The assembled EC* complex was applied to a Superose 6 Increase 3.2/300 column (Cytiva) equilibrated in K50 buffer (20 mM HEPES, pH 7.5, 50 mM KCl, 4 mM MgCl2, 1 mM DTT). The peak fraction of EC* was incubated with a 2× molar excess of RECQL5 and 1 mM ADP on ice for 30 min, cross-linked with 0.05% (vol/vol) glutaraldehyde for 45 min on ice and used directly for grid freezing.
The EC–TCR–RECQL5 complex was formed on a DNA–RNA scaffold with a mismatch bubble of 15 nucleotides43: template DNA, 5′-CGCTCTGCTCCTTCTCCCATCCTCTCGATGGCTATGAGATCAACTAG-3′; non-template DNA, 5′-CTAGTTGATCTCATATTTCATTCCTACTCAGGAGAAGGAGCAGAGCG-3′; RNA, 5′-ACAUCAUAACAUUUGAACAAGAAUAUAUAUACAAAAUCGAGAGGA-3′. The DNA and RNA oligonucleotides were synthesized by IDT. RNA was annealed with equimolar template DNA by incubating at 90 °C for 2 min, followed by a gradual decrease in temperature at a rate of 1 °C min−1 to a final temperature of 30 °C in 20 mM HEPES, pH 7.5 and 100 mM NaCl. Porcine Pol II (75 pmol) was incubated with the RNA–DNA hybrid (150 pmol) at 30 °C for 10 min, followed by the addition of the non-template DNA (300 pmol) and incubation at 30 °C for an additional 10 min. The complex was mixed with elongation factors (SPT6 and PAF, 150 pmol each) and phosphorylated with P-TEFb (25 pmol) and ATP (1 mM) in SEC100 buffer for 30 min at 30 °C before being subject to a Superose 6 Increase 3.2/300 column (Cytiva) equilibrated in K50 buffer. Peak fraction was incubated with a 2× molar excess of RECQL5 on ice for 30 min, followed by incubation with a 2× molar excess of TCR components (CSB, CSA–DDB1 and UVSSA), a 4× molar excess of ELOF1 and 1 mM ADP at 30 °C for 10 min. The assembled EC–TCR–RECQL5 complex was cross-linked with 0.05% (vol/vol) glutaraldehyde for 45 min on ice and used directly for grid freezing.
The sample (2.5 μl) was applied to R3.5/1 carbon grids (Quantifoil) with a continuous carbon support (~2.5 nm) for the EC–RECQL5 and EC*–RECQL5 complexes and no support layer for the EC–TCR–RECQL5 complex. The grids were glow discharged for 13–15 s with the Sputter Coater S150B (Edwards Vacuum). The grids were incubated with the sample for 30 s and blotted for 1–1.5 s before plunge-freezing in liquid ethane with a Vitrobot Mark IV (Thermo Fisher) operated at 4 °C and 100% humidity.
Pulldown assay
For pulldown of non-phosphorylated Pol II with different RECQL5 constructs, Twin-Strep-tagged human Pol II (12 pmol) was incubated with wild-type or mutant RECQL5 (36 pmol) on ice for 30 min in G-SEC150 buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 3 mM MgCl2, 5% glycerol, 1 mM DTT). Samples were incubated with Strep-Tactin XT 4Flow high-capacity resin (IBA) at 4 °C for 2 h. The resin was washed with G-SEC150 buffer and eluted with G-SEC150 buffer supplemented with 50 mM biotin. The eluate was separated on a 4–12% NuPAGE Bis-Tris gel (Invitrogen) and stained with InstantBlue (Abcam). Pulldowns using phosphorylated Pol II with different RECQL5 constructs were performed in the same way except that Pol II was incubated with P-TEFb (4 pmol) and ATP (1 mM) in SEC100 buffer for 30 min at 30 °C before mixing with RECQL5 constructs in G-SEC150 buffer.
For pulldown of the Pol II CTD with RECQL5SRI, His6-MBP-tagged Pol II CTD (100 pmol) was phosphorylated with P-TEFb (25 pmol) and ATP (1 mM) in SEC100 buffer for 30 min at 30 °C. P-TEFb storage buffer (20 mM HEPES, pH 7.5, 300 mM NaCl, 10% glycerol, 1 mM DTT) was used instead of P-TEFb as a negative control for the non-phosphorylated CTD condition. The CTD was incubated with the SRI domain of RECQL5 (500 pmol) on ice for 30 min, followed by incubation with amylose resin (NEB) equilibrated in G-SEC150 buffer at 4 °C for 1 h. The resin was washed with G-SEC150 buffer and eluted with 20 mM maltose in G-SEC150 buffer. The eluate was loaded on a 4–12% NuPAGE Bis-Tris gel (Invitrogen) and stained with InstantBlue (Abcam).
For pulldown of EC or EC* with RECQL5, the elongation complex was prepared in the same way as described for sample preparation for cryo-EM, except that Twin-Strep-tagged human Pol II was used. Final protein amounts used for complex formation were 12 pmol human Pol II, 24 pmol RNA–DNA hybrid and 48 pmol non-template DNA. The phosphorylation reaction was performed with P-TEFb (4 pmol) and ATP (1 mM) in SEC100 buffer for 30 min at 30 °C for either Pol II alone or with elongation factors (SPT6, DSIF and PAF, 24 pmol each). Samples were incubated with Strep-Tactin XT 4Flow high-capacity resin (IBA) at 4 °C for 2 h. The resin was washed with SEC100 buffer and eluted with 50 mM biotin in SEC100 buffer. The eluate was separated on a 4–12% NuPAGE Bis-Tris gel (Invitrogen) and stained with InstantBlue (Abcam).
For pulldown of RECQL5 with the elongation factors SPT6, DSIF, PAF and RTF1, elongation factors (100 pmol) were phosphorylated with P-TEFb (17 pmol) and ATP (1 mM) in SEC100 buffer for 30 min at 30 °C, followed by addition of His6-MBP-tagged RECQL5 (50 pmol) and incubation on ice for 30 min. Samples were incubated with amylose resin (NEB) equilibrated in K50 buffer at 4 °C for 1 h. The resin was washed with K50 buffer and eluted with K50 buffer supplemented with 20 mM maltose. The proteins were separated on a 4–12% NuPAGE Bis-Tris gel (Invitrogen) and stained with InstantBlue (Abcam).
RNA extension assay
All RNA extension assays were performed with a complementary DNA scaffold as follows: template DNA, 5′-CTGGACTACTGCGCCCTAGACGTGCAGCAAGCTTGGGCTGCAGGTAACCAGTTCTACATGCTAGATACTTACCTGGTCGGAGGCCGACGG-3′; non-template DNA, 5′-CCGTCGGCCTCCGACCAGGTAAGTATCTAGCATGTAGAACTGGTTACCTGCAGCCCAAGCTTGCTGCACGTCTAGGGCGCAGTAGTCCAG-3′; RNA, 5′-/6-FAM/-UUUUUUCCAGGUAAG-3′. All oligonucleotides were synthesized by IDT and resuspended in water.
RNA was annealed with an equimolar amount of template DNA by incubating at 90 °C for 2 min, followed by a gradual decrease in temperature at a rate of 1 °C min−1 to a final temperature of 30 °C in 20 mM HEPES, pH 7.5 and 100 mM NaCl. All concentrations refer to the final concentrations used in the assay. Porcine Pol II (150 nM) was incubated with the RNA–DNA hybrid (100 nM) at 30 °C for 10 min, followed by the addition of the non-template DNA (200 nM) and incubation at 30 °C for an additional 10 min. The complex was mixed with elongation factors (SPT6, DSIF and PAF, 150 nM each) and phosphorylated with P-TEFb (100 nM) and ATP (1 mM) in 20 mM HEPES, pH 7.5, 100 mM NaCl, 3 mM MgCl2, 4% glycerol and 1 mM DTT at 30 °C for 30 min. The reactions were incubated with wild-type or mutant RECQL5 (1.5 µM) on ice for 30 min. Transcription extension was started by adding 100 μM NTP at 20 °C. The reactions were quenched at various time points in equal volume of 2× stop buffer (6.4 M urea, 50 mM EDTA, pH 8.0, 1× TBE). Reactions were treated with 0.13 unit per μl proteinase K (NEB) for 20 min at 37 °C and applied onto 15% denaturing gels (15% acrylamide/Bis-acrylamide 19:1, 7 M urea and 1× TBE, run in 0.5× TBE at 200 V for 100 min). The gels were visualized using the 6-FAM label on the RNA with the Typhoon FLA 9500 Imager (GE Healthcare). Extension assays with additional TCR factors were performed in the same way with the exception that EC* was incubated with 0.75 µM RECQL5, and 0.75 µM TCR factors were added to the reaction after extension by EC*–RECQL5 for 2 min.
Electromobility shift assay
Electromobility shift assays for wild-type and D157A mutant RECQL5 were performed with two types of DNA scaffolds, one with a 3′ overhang and one with a splayed duplex. DNA was annealed in 20 mM HEPES, pH 7.5 and 100 mM NaCl by heating at 94 °C for 4 min and slowly cooling down to 30 °C at a rate of 1 °C min−1. Double-stranded DNA at a final concentration of 0.5 μM was mixed with 0–2.5 μM of wild-type or D157A RECQL5 in 20 mM HEPES, pH 7.5, 66 mM NaCl, 5% glycerol, 1 mM DTT and 0.25 mg ml−1 BSA and incubated on ice for 30 min. The samples were then mixed with Novex Hi-Density TBE Sample Buffer (Thermo Fisher) and loaded onto a 6% DNA retardation gel (Invitrogen). Electrophoresis was performed at 4 °C and 100 V in 0.5× TBE buffer for 105 min. Gels were stained with SYBR Gold (Thermo Fisher) and imaged with a Typhoon FLA 9500 Imager (GE Healthcare).
Pol II ubiquitination assay
Pol II elongation complexes were formed on the same DNA–RNA scaffold as that for the cryo-EM studies of the EC–TCR–RECQL5 complex. RNA was annealed with equimolar template DNA by incubating at 60 °C for 5 min, followed by a gradual decrease in temperature at a rate of 1 °C min−1 to a final temperature of 30 °C in 20 mM HEPES, pH 7.5, 100 mM NaCl and 3 mM MgCl2. All concentrations refer to the final concentrations used in the assay. Human Pol II (150 nM) was incubated with the RNA–DNA hybrid (300 nM) at 30 °C for 10 min, followed by addition of the non-template DNA (600 nM) and incubation at 30 °C for another 10 min. Subsequently, RECQL5 constructs (0 or 450 nM) were added to the elongation complex and incubated on ice for 30 min before adding CSB (300 nM), CSA–DDB1 (300 nM), CUL4A–RBX1 (300 nM), ELOF1 (600 nM) and UVSSA (360 nM) for a further incubation at 30 °C for 15 min. Ubiquitination reactions were initiated upon addition of UBE1 (150 nM, R&D Systems), UbcH5b (1.75 μM), ubiquitin (150 μM, R&D Systems) and ATP (2 mM) in 50 mM Tris, pH 8.0, 50 mM NaCl, 10 mM MgCl2 and 1 mM DTT at 37 °C. The reactions were quenched at various time points by mixing with 4× SDS loading dye (20 mM Tris-HCl, pH 6.6, 8% SDS, 40% glycerol, 0.8% bromophenol blue and 400 mM DTT). Reactions were loaded on a 3–8% Tris-acetate gel (Invitrogen) and transferred onto a 0.2-µm nitrocellulose membrane (Cytiva). The membrane was blocked with 5% (wt/vol) milk in PBS for 30 min at room temperature and incubated with F-12 anti-RPB1 antibody (1:1,000 dilution, Santa Cruz Biotechnology, sc-55492) or anti-CSB antibody (1:1,000 dilution, Santa Cruz Biotechnology, sc-166042) overnight at 4 °C. The membranes were washed with PBST (PBS with 0.2% Tween-20) and incubated with HRP-conjugated anti-mouse secondary antibody (1:10,000 dilution, Proteintech, SA00001-1) for 45 min at room temperature. The membranes were washed with PBST, developed with the Pierce ECL chemiluminescent substrate (Thermo Fisher) and filmed with an OPTIMAX processor (Protec).
Competition assay
Pol II elongation complex was prepared in the same way as described for the ubiquitination assays. Final protein amounts used for complex formation were 12 pmol human Pol II, 24 pmol RNA–DNA hybrid and 48 pmol non-template DNA. The elongation complex was either assembled using non-phosphorylated Pol II or phosphorylated with 4 pmol P-TEFb and 1 mM ATP in SEC100 buffer for 30 min at 30 °C. The assembled elongation complex was either incubated with wild-type RECQL5 or RECQL5ΔSRI (36 pmol) first on ice for 20 min and then with the TCR complex (36 pmol CSB and CSA–DDB1, 50 pmol UVSSA and ELOF1) on ice for 20 min in SEC100 buffer or vice versa. His6-MBP-tagged wild-type RECQL5 was used to avoid band overlapping with DDB1 in the competition assay using non-phosphorylated Pol II. RECQL5ΔSRI was used in the competition assay with phosphorylated Pol II to eliminate the SRI–CTD interaction and focus on the competition at the Pol II jaw. The elongation complex was incubated with RECQL5 (36 pmol) alone in SEC100 buffer on ice for 20 min as a positive control. The complexes were incubated with Strep-Tactin XT 4Flow high-capacity resin (IBA) at 4 °C for 2 h. The resin was washed with SEC100 buffer with 5% glycerol and eluted with 50 mM biotin in SEC100 buffer with 5% glycerol. The eluate was separated on a 4–12% NuPAGE Bis-Tris gel (Invitrogen) and stained with InstantBlue (Abcam).
Cryo-EM data collection and processing
Cryo-EM data were collected on the 300-kV Titan Krios with a Falcon 4i direct electron detector (Thermo Fisher). Automated data acquisition was performed with EPU (Thermo Fisher) at a nominal magnification of ×96,000 (0.8156 Å per pixel). Image stacks of 40 frames were collected with a defocus range of −0.5 μm to −2.0 μm in electron-counting mode and at a dose rate of 0.92–1.06 e− Å−2 per frame. A total of 8,602 image stacks were collected for the EC–RECQL5 complex. A total of 60,808 image stacks were collected across two datasets for the EC*–RECQL5 complex. A total of 11,156 image stacks were collected for the EC–TCR–RECQL5 complex.
Motion correction and estimation of the contrast transfer function (CTF) were done in RELION 5.0 (refs. 63,64). Particles in 400 pixels × 400 pixels for EC–RECQL5 and in 480 pixels × 480 pixels for EC*–RECQL5 and EC–TCR–RECQL5 were selected by automatic particle picking in Warp65. For EC–RECQL5 and EC*–RECQL5, further processing steps were performed in RELION 5.0 with final local refinement in cryoSPARC66. Further processing steps for EC–TCR–RECQL5 were performed in cryoSPARC.
For the EC–RECQL5 complex, two-dimensional (2D) classification, followed by three-dimensional (3D) refinement and 3D classification with fine-angle sampling, was performed to remove bad particles from the dataset (Extended Data Fig. 2a,b). Signal subtraction with a soft mask near the Pol II jaw (where the extra density of RECQL5 is) followed by focused 3D classification without alignment was performed to separate EC–RECQL5 particles from EC-only particles (Extended Data Fig. 2c,d). Particles containing densities corresponding to RECQL5 were reverted to original particles and 3D refined, followed by CTF refinement with per-particle defocus estimation and Bayesian polishing to correct beam-induced particle motion (Extended Data Fig. 2e). Polished particles were subjected to an additional round of signal subtraction and focused 3D classification on RECQL5 to further remove the EC-only particles (Extended Data Fig. 2f). Subsequent signal subtraction with a soft mask on the downstream DNA and focused 3D classification without alignment were performed to exclude any apo Pol II–RECQL5 particles (Extended Data Fig. 2g,h). Particles containing densities of downstream DNA were reverted to original particles and imported into cryoSPARC for local refinement, resulting in the final EC–RECQL5 reconstruction with 66,771 particles at an overall resolution of 2.8 Å (Extended Data Fig. 2i).
Extended Data Fig. 2. Cryo-EM data processing of the EC-RECQL5 complex.
After removing junk particles with 2D-classification, the remaining particles were 3D-refined and further cleaned up using local 3D-classification (a, b). Good particles were extracted without binning and 3D-refined (c). Following signal subtraction with a soft mask near the Pol II jaw and focused 3D-classification without alignment (d), particles containing densities of RECQL5 were reverted to original particles and 3D-refined before CTF refinement and Bayesian polishing (e). A second round of signal subtraction and focused 3D-classification on RECQL5 were performed to further remove the EC-only particles (f, g). Subsequent signal subtraction with a soft mask on the downstream DNA and focused 3D-classification were performed to exclude the apo Pol II-RECQL5 particles (h). This resulted in a final EC-RECQL5 reconstruction at 2.8 Å resolution (i). The cryo-EM densities were coloured according to Fig. 1d. j, Gold-standard Fourier Shell Correlation (FSC) curves of the overall map of EC-RECQL5.
For the two datasets of the EC*–RECQL5 complex, particles after cleanup in 2D and 3D classification were CTF refined and Bayesian polished separately (Extended Data Fig. 3a–c,e–g). Polished particles in dataset 2 were further cleaned up by an additional round of 3D classification with fine-angle sampling (Extended Data Fig. 3h,i). Signal subtraction with a soft mask on the RECQL5 IRI domain and focused 3D classification without alignment were performed to separate EC*–RECQL5 particles from EC*-only particles for both datasets (Extended Data Fig. 3d,j). Particles containing densities corresponding to RECQL5 from both datasets were combined, reverted to original particles and 3D refined (Extended Data Fig. 3k). Following signal subtraction with a soft mask on PAF and focused 3D classification without alignment (Extended Data Fig. 3l), particles containing PAF densities were selected, reverted to original particles and imported into cryoSPARC for local refinement. This resulted in the final EC*–RECQL5 reconstruction with 314,016 particles at an overall resolution of 2.0 Å (Extended Data Fig. 3m).
Extended Data Fig. 3. Cryo-EM data processing of the EC*-RECQL5 complex.
2D-classification, followed by 3D-refinement and local 3D-classification were performed to remove bad particles from the datasets (a, b, e, f). Remaining particles underwent CTF refinement and Bayesian polishing separately (c, g). Polished particles in Dataset 2 were subjected to an additional round of local 3D-classification to exclude junk particles (h, i). Following signal subtraction of Pol II and focused 3D-classification on RECQL5 (d, j), particles containing densities of RECQL5 from both datasets were combined, reverted to original particles and 3D-refined (k). This is followed by signal subtraction and focused 3D-classification with a soft mask on PAF (l). Particles containing densities of PAF were reverted to original particles and 3D-refined, resulting in a final EC*-RECQL5 reconstruction at an overall resolution of 2.0 Å (m). Particle subtraction followed by local refinement improved the local resolution of SPT6core, SPT6core-Pol IIstalk and PAF (CTR9-SKI8 region) (n-p). Signal subtraction and focused 3D-classification without alignment on SPT6tSH2 identified a previously unassigned density of CDC73 bound to the tSH2 domain of SPT6 (q). The cryo-EM densities were coloured according to Fig. 1e.
To improve the resolution of elongation factors, soft masks were applied individually onto SPT6core, SPT6core–Pol IIstalk, SPT6tSH2, PAF (CTR9–SKI8 region), PAF1–LEO1, SPT4 and SPT5 (KOW2–KOW3 region). Following particle subtraction, 3D classification without alignment was performed for SPT6tSH2, PAF1–LEO1, SPT4 and SPT5 (KOW2–KOW3 region), resulting in medium-resolution maps of each region. These maps allowed docking of existing structures and AlphaFold67-predicted models of the elongation factors into respective regions. For SPT6core, SPT6core–Pol IIstalk and PAF (CTR9–SKI8 region), 3D classification did not find any classes lacking densities corresponding to the elongation factors. Therefore, all subtracted particles were imported into cryoSPARC for local refinement, resulting in a resolution of 2.5 Å for all three maps of SPT6core, SPT6core–Pol IIstalk and PAF (CTR9–SKI8 region) (Extended Data Fig. 3n–p).
For the dataset of the EC–TCR–RECQL5 complex, particles after cleanup in 2D classification were CTF refined (Extended Data Fig. 9a,b), followed by focused 3D classification without alignment using a soft mask on the RECQL5 IRI domain to separate EC–TCR–RECQL5 particles from EC–TCR-only particles (Extended Data Fig. 9c). Particles containing densities of RECQL5 were selected and 3D refined (Extended Data Fig. 9d). Subsequent focused 3D classification without alignment using a soft mask on TCR was performed to exclude EC–RECQL5-only particles (Extended Data Fig. 9e). This resulted in the final EC–TCR–RECQL5 reconstruction with 19,458 particles at an overall resolution of 3.5 Å (Extended Data Fig. 9f).
Local resolution of the maps was estimated using cryoSPARC except for the EC*–RECQL5 and EC–TCR–RECQL5 overall maps, which were estimated in RELION to obtain locally filtered and sharpened maps. All resolution calculations were based on gold-standard FSC using the FSC = 0.143 criterion. A summary of all EM reconstructions obtained in this paper is shown in Table 1.
Model building and refinement
Initial models of S. scrofa Pol II (PDB 7B0Y, ref. 35) and EC* (PDB 6GMH, ref. 2) as well as AlphaFold67 predictions of RECQL5 and elongation factors were rigid body fitted into the overall map in Chimera68. The Pol II and RECQL5 models were manually adjusted in Coot69 using the sharpened overall map of EC*–RECQL5 (2.0 Å), and the structure was real-space refined in PHENIX70. The SPT6core model was manually adjusted in Coot using the sharpened local refined SPT6 map (2.5 Å) and real-space refined in PHENIX. The refined SPT6core model was then fitted into the sharpened local refined SPT6core–Pol IIstalk map (2.5 Å), adjusted in Coot and real-space refined in PHENIX. The PAF model was manually adjusted in Coot using the sharpened local refined PAF map (2.5 Å), and the structure was real-space refined in PHENIX. The rest of the elongation factors were rigid body docked into the overall map in Chimera. The resulting complete model of EC*–RECQL5 was then real-space refined in the locally filtered and sharpened overall map using PHENIX and structure restraints of Pol II, SPT6core–Pol IIstalk and PAF.
An additional density was found to bind the tSH2 domain of SPT6, and the tSH2 domain was in a horizontal conformation compared to previous EC* structures2 (Extended Data Fig. 3q). De novo model building using ModelAngelo71 identified this unassigned density to be CDC73. This allowed us to identify a previously uncharacterized interface between the tSH2 domain of SPT6 and CDC73 of PAF (Extended Data Fig. 5e), consistent with biochemical analysis in yeast72.
The EC–RECQL5 model was fitted into the sharpened overall map of EC–RECQL5, manually adjusted in Coot and real-space refined in PHENIX.
The EC–RECQL5 model from EC*–RECQL5 and the TCR model (8B3D)38 were rigid body fitted into the sharpened overall map of EC–TCR–RECQL5 and real-space refined using ADP refinement and the locally filtered and sharpened overall map in PHENIX.
Figures were generated using PyMOL (PyMOL Molecular Graphics System, version 2.0, Schrödinger) and ChimeraX73. Sequence alignment was performed using Jalview74.
Cross-linking-coupled mass spectrometry
Samples for cross-linking-coupled mass spectrometry were prepared essentially as described for cryo-EM studies of EC*–RECQL5, except that peak fractions of EC* were combined and incubated with a 1.5× molar excess of RECQL5 on ice for 30 min, cross-linked with 1 mM BS3 on ice for 1 h and quenched with 50 mM Tris-HCl, pH 8.0.
The quenched solution was reduced with 5 mM DTT and alkylated with 20 mM iodoacetamide. The SP3 protocol as described in refs. 75,76 was used to clean up and buffer exchange the reaction. Briefly, the complex was washed with ethanol, resuspended in 100 mM NH4HCO3 and digested with trypsin (Promega) at an enzyme-to-substrate ratio of 1:20 and with 0.1% ProteaseMAX (Promega) overnight at 37 °C. Digested peptides were purified using HyperSep SpinTip P-20 C18 columns (Thermo Scientific), eluted with 60% (vol/vol) acetonitrile (ACN) and dried using a Speed Vac Plus concentrator (Savant). Dried peptides were resuspended in 30% (vol/vol) ACN and separated using a Superdex 30 Increase 3.2/300 column (Cytiva) at a flow rate of 10 μl min−1 in 30% (vol/vol) ACN and 0.1% (vol/vol) trifluoroacetic acid. Fractions containing cross-linked peptides were collected and dried with the Speed Vac Plus concentrator (Savant). Dried peptides were suspended in 3% (vol/vol) ACN and 0.1% (vol/vol) formic acid and analyzed by nanoscale capillary LC–MS/MS using an Ultimate U3000 HPLC system (Thermo Scientific) to deliver a flow of 300 nl min−1. Peptides were trapped on a C18 Acclaim PepMap 100, 5-μm, 0.3-μm × 5-mm cartridge (Thermo Scientific) before separation on an Aurora Ultimate C18, 1.7-μm, 75-μm × 25-cm column (IonOpticks). Peptides were eluted on a 90-min gradient and interfaced via an EASY-Spray ionization source to a Q Exactive Plus mass spectrometer (Thermo Scientific). Data were acquired in data-dependent mode using a Top-15 method, in which high-resolution full-mass scans were carried out (R = 70,000, m/z 400–1,500) followed by a higher-energy collision dissociation of 30 V. The tandem mass spectra were recorded (R = 60,000, automatic gain control target = 5 × 105, maximum injection time = 100 ms, isolation window = 1.2 m/z, dynamic exclusion = 40 s).
Xcalibur raw files were converted to MGF files using ProteoWizard77, and cross-links were analyzed with xiSEARCH78. Search conditions used a maximum of three missed cleavages with a minimum peptide length of 5. Variable modifications used were carbamidomethylation of cysteine (57.02146 Da) and oxidation of methionine (15.99491 Da). The false discovery rate was set to 5%. The sequence database was assembled from all proteins within the complex. Cross-link sites were visualized with xiVIEW79 and the PyXlinkViewer plugin80 in PyMOL version 2.0.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41594-025-01586-6.
Supplementary information
RECQL5 and the TCR complex coordinate to regulate the transcription elongation rate.
Source data
Unprocessed gels.
Unprocessed gels.
Unprocessed gels.
Unprocessed gels and western blots.
Unprocessed gels.
Unprocessed gels.
Unprocessed gels.
Statistical source data.
Unprocessed gels and western blots.
Acknowledgements
We thank all members of the Zhang laboratory for discussion, particularly G. A. Arroyo for participating in the initial purification of RECQL5. We thank the LMB EM facility and scientific computing for maintaining the microscopes and the computing cluster, respectively, and K. Turton for maintaining the insect cell facility. We thank S. Aibara for suggestions on model building and the manuscript and D. Barford, L. Passmore and M. Hegde for suggestions on the manuscript. This work was funded by the Medical Research Council, as part of United Kingdom Research and Innovation (also known as UK Research and Innovation) with the MRC file reference number MC_UP_1201/30 for S.Z. For open access, the MRC Laboratory of Molecular Biology has applied a CC BY public copyright license to any author accepted manuscript version arising. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Extended data
Extended Data Fig. 4. Local resolution estimation and FSC curves of EC*-RECQL5 maps.
a, Gold-standard FSC curves of the overall map of EC*-RECQL5. b, Local resolution estimations of the locally filtered and sharpened overall map in both surface and slice views. RECQL5 density is highlighted with dashed pink lines. c-h, FSC curves of the focused refined maps of SPT6Core-Pol IIStalk (c), SPT6Core (e) and PAF (g). Local resolution estimation of the non-sharpened focused refined maps showed improved density of SPT6Core-Pol IIStalk (d), SPT6Core (f) and PAF (h) to mostly beyond 2.5 Å. i, Local resolution estimation of the RECQL5-Pol II interface in the sharpened overall map. The brake helix interface with the Pol II jaw is resolved to 3 Å, revealing bulky side chain densities. The KIX domain is more mobile, at around 4-4.5 Å. RECQL5 density is highlighted with dashed pink lines.
Author contributions
L.Z. and S.Z. designed and performed experiments, and collected and analyzed cryo-EM data. Y.G. performed experiments. T.M. and C.F. performed mass spectrometry. A.T.V. and S.Z. initiated the project. S.Z. supervised the project and built models. S.Z. and L.Z. wrote the paper with input from all other authors.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Hauke Hillen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Data availability
The cryo-EM reconstructions and final models were deposited in the EMDB under accession code EMD-52440 and the PDB under accession code 9HVO for the EC–RECQL5 complex, EMD-52443 (overall), EMD-52441 (SPT6 stalk), EMD-52442 (PAF) and PDB 9HVQ for the EC*–RECQL5 complex, and EMD-52449 and PDB 9HWG for the EC–TCR–RECQL5 complex. The raw data for cross-linking-coupled mass spectrometry of the EC*–RECQL5 complex are available at PRIDE PXD062042. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41594-025-01586-6.
Supplementary information
The online version contains supplementary material available at 10.1038/s41594-025-01586-6.
References
- 1.Peterlin, B. M. & Price, D. H. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell23, 297–305 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Vos, S. M. et al. Structure of activated transcription complex Pol II–DSIF–PAF–SPT6. Nature560, 607–612 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Muniz, L., Nicolas, E. & Trouche, D. RNA polymerase II speed: a key player in controlling and adapting transcriptome composition. EMBO J.40, e105740 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonkers, I. & Lis, J. T. Getting up to speed with transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol.16, 167–177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saldi, T., Riemondy, K., Erickson, B. & Bentley, D. L. Alternative RNA structures formed during transcription depend on elongation rate and modify RNA processing. Mol. Cell81, 1789–1801 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslanzadeh, V., Huang, Y., Sanguinetti, G. & Beggs, J. D. Transcription rate strongly affects splicing fidelity and cotranscriptionality in budding yeast. Genome Res.28, 203–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotsantis, P. et al. Increased global transcription activity as a mechanism of replication stress in cancer. Nat. Commun.7, 13087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Mata, M. et al. A slow RNA polymerase II affects alternative splicing in vivo. Mol. Cell12, 525–532 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Dujardin, G. et al. How slow RNA polymerase II elongation favors alternative exon skipping. Mol. Cell54, 683–690 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Gromak, N., West, S. & Proudfoot, N. J. Pause sites promote transcriptional termination of mammalian RNA polymerase II. Mol. Cell. Biol.26, 3986–3996 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazelbaker, D. Z., Marquardt, S., Wlotzka, W. & Buratowski, S. Kinetic competition between RNA polymerase II and Sen1-dependent transcription termination. Mol. Cell49, 55–66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander, R. D., Innocente, S. A., Barrass, J. D. & Beggs, J. D. Splicing-dependent RNA polymerase pausing in yeast. Mol. Cell40, 582–593 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, R. M., Rino, J., Carvalho, C., Kirchhausen, T. & Carmo-Fonseca, M. Live-cell visualization of pre-mRNA splicing with single-molecule sensitivity. Cell Rep.4, 1144–1155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonkers, I., Kwak, H. & Lis, J. T. Genome-wide dynamics of Pol II elongation and its interplay with promoter proximal pausing, chromatin, and exons. eLife3, e02407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saponaro, M. et al. RECQL5 controls transcript elongation and suppresses genome instability associated with transcription stress. Cell157, 1037–1049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aygun, O. et al. Direct inhibition of RNA polymerase II transcription by RECQL5. J. Biol. Chem.284, 23197–23203 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassube, S. A., Jinek, M., Fang, J., Tsutakawa, S. & Nogales, E. Structural mimicry in transcription regulation of human RNA polymerase II by the DNA helicase RECQL5. Nat. Struct. Mol. Biol.20, 892–899 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izumikawa, K. et al. Association of human DNA helicase RecQ5β with RNA polymerase II and its possible role in transcription. Biochem. J.413, 505–516 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Bernstein, K. A., Gangloff, S. & Rothstein, R. The RecQ DNA helicases in DNA repair. Annu. Rev. Genet.44, 393–417 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aygun, O., Svejstrup, J. & Liu, Y. A RECQ5–RNA polymerase II association identified by targeted proteomic analysis of human chromatin. Proc. Natl Acad. Sci. USA105, 8580–8584 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, Y. et al. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev.21, 3073–3084 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, Y. et al. Recql5 and Blm RecQ DNA helicases have nonredundant roles in suppressing crossovers. Mol. Cell. Biol.25, 3431–3442 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrs, M., Hasanova, Z., Oravetzova, A., Dobrovolna, J. & Janscak, P. RECQ5: a mysterious helicase at the interface of DNA replication and transcription. Genes11, 232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwendener, S. et al. Physical interaction of RECQ5 helicase with RAD51 facilitates its anti-recombinase activity. J. Biol. Chem.285, 15739–15745 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng, L. et al. MRE11 complex links RECQ5 helicase to sites of DNA damage. Nucleic Acids Res.37, 2645–2657 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanagaraj, R., Saydam, N., Garcia, P. L., Zheng, L. & Janscak, P. Human RECQ5β helicase promotes strand exchange on synthetic DNA structures resembling a stalled replication fork. Nucleic Acids Res.34, 5217–5231 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamadeh, Z. & Lansdorp, P. RECQL5 at the intersection of replication and transcription. Front. Cell Dev. Biol.8, 324 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, H. & Davis, A. J. Human RecQ helicases in DNA double-strand break repair. Front. Cell Dev. Biol.9, 640755 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kizer, K. O. et al. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol. Cell. Biol.25, 3305–3316 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanagaraj, R. et al. RECQ5 helicase associates with the C-terminal repeat domain of RNA polymerase II during productive elongation phase of transcription. Nucleic Acids Res.38, 8131–8140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebehmed, J., Revy, P., Faure, G., de Villartay, J. P. & Callebaut, I. Expanding the SRI domain family: a common scaffold for binding the phosphorylated C-terminal domain of RNA polymerase II. FEBS Lett.588, 4431–4437 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Islam, M. N., Fox, D. 3rd, Guo, R., Enomoto, T. & Wang, W. RecQL5 promotes genome stabilization through two parallel mechanisms—interacting with RNA polymerase II and acting as a helicase. Mol. Cell. Biol.30, 2460–2472 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsin, J. P. & Manley, J. L. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev.26, 2119–2137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidemann, M., Hintermair, C., Voss, K. & Eick, D. Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription. Biochim. Biophys. Acta1829, 55–62 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Zhang, S. et al. Structure of a transcribing RNA polymerase II–U1 snRNP complex. Science371, 305–309 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Farnung, L., Ochmann, M., Garg, G., Vos, S. M. & Cramer, P. Structure of a backtracked hexasomal intermediate of nucleosome transcription. Mol. Cell82, 3126–3134 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Cheung, A. C. & Cramer, P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature471, 249–253 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Kokic, G. et al. Structural basis for RNA polymerase II ubiquitylation and inactivation in transcription-coupled repair. Nat. Struct. Mol. Biol.31, 536–547 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Weegen, Y. et al. ELOF1 is a transcription-coupled DNA repair factor that directs RNA polymerase II ubiquitylation. Nat. Cell Biol.23, 595–607 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu, J. et al. Structural basis for the initiation of eukaryotic transcription-coupled DNA repair. Nature551, 653–657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selby, C. P. & Sancar, A. Cockayne syndrome group B protein enhances elongation by RNA polymerase II. Proc. Natl Acad. Sci. USA94, 11205–11209 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lake, R. J. et al. The sequence-specific transcription factor c-Jun targets Cockayne syndrome protein B to regulate transcription and chromatin structure. PLoS Genet.10, e1004284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kokic, G., Wagner, F. R., Chernev, A., Urlaub, H. & Cramer, P. Structural basis of human transcription–DNA repair coupling. Nature598, 368–372 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, K. B., Wang, D., Lippard, S. J. & Sharp, P. A. Transcription-coupled and DNA damage-dependent ubiquitination of RNA polymerase II in vitro. Proc. Natl Acad. Sci. USA99, 4239–4244 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Weegen, Y. et al. The cooperative action of CSB, CSA, and UVSSA target TFIIH to DNA damage-stalled RNA polymerase II. Nat. Commun.11, 2104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakazawa, Y. et al. Ubiquitination of DNA damage-stalled RNAPII promotes transcription-coupled repair. Cell180, 1228–1244 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Tufegdzic Vidakovic, A. et al. Regulation of the RNAPII pool is integral to the DNA damage response. Cell180, 1245–1261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groisman, R. et al. CSA-dependent degradation of CSB by the ubiquitin-proteasome pathway establishes a link between complementation factors of the Cockayne syndrome. Genes Dev.20, 1429–1434 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maslon, M. M. et al. A slow transcription rate causes embryonic lethality and perturbs kinetic coupling of neuronal genes. EMBO J.38, e101244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma, A. et al. Calcium-mediated histone modifications regulate alternative splicing in cardiomyocytes. Proc. Natl Acad. Sci. USA111, E4920–E4928 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muniz, L. et al. Control of gene expression in senescence through transcriptional read-through of convergent protein-coding genes. Cell Rep.21, 2433–2446 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Schor, I. E., Fiszbein, A., Petrillo, E. & Kornblihtt, A. R. Intragenic epigenetic changes modulate NCAM alternative splicing in neuronal differentiation. EMBO J.32, 2264–2274 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, M. et al. SUMO2 conjugation of PCNA facilitates chromatin remodeling to resolve transcription–replication conflicts. Nat. Commun.9, 2706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li, M., Xu, X. & Liu, Y. The SET2–RPB1 interaction domain of human RECQ5 is important for transcription-associated genome stability. Mol. Cell. Biol.31, 2090–2099 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balajee, A. S., May, A., Dianov, G. L., Friedberg, E. C. & Bohr, V. A. Reduced RNA polymerase II transcription in intact and permeabilized Cockayne syndrome group B cells. Proc. Natl Acad. Sci. USA94, 4306–4311 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tantin, D., Kansal, A. & Carey, M. Recruitment of the putative transcription–repair coupling factor CSB/ERCC6 to RNA polymerase II elongation complexes. Mol. Cell. Biol.17, 6803–6814 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Gool, A. J. et al. The Cockayne syndrome B protein, involved in transcription-coupled DNA repair, resides in an RNA polymerase II-containing complex. EMBO J.16, 5955–5965 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ariza, A. J. F. et al. Structural insights into transcriptional regulation by the helicase RECQL5. Preprint at bioRxiv10.1101/2025.01.29.634372 (2025). [DOI] [PMC free article] [PubMed]
- 59.Sebesta, M. et al. Mechanisms of transcription attenuation and condensation of RNA polymerase II by RECQ5. Preprint at bioRxiv10.1101/2025.02.05.636647 (2025).
- 60.Gradia, S. D. et al. MacroBac: new technologies for robust and efficient large-scale production of recombinant multiprotein complexes. Methods Enzymol.592, 1–26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu, H. & Naismith, J. H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol.8, 91 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, L. et al. Structure of a transcribing Pol II–DSIF–SPT6–U1 snRNP complex. Preprint at bioRxiv10.1101/2025.03.21.644610 (2025). [DOI] [PMC free article] [PubMed]
- 63.Scheres, S. H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol.415, 406–418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheres, S. H. Processing of structurally heterogeneous cryo-EM data in RELION. Methods Enzymol.579, 125–157 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Tegunov, D. & Cramer, P. Real-time cryo-electron microscopy data preprocessing with Warp. Nat. Methods16, 1146–1152 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem.25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr.66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol.75, 861–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature628, 450–457 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellison, M. A. et al. Spt6 directly interacts with Cdc73 and is required for Paf1 complex occupancy at active genes in Saccharomyces cerevisiae. Nucleic Acids Res.51, 4814–4830 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci.27, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waterhouse, A. M., Procter, J. B., Martin, D. M., Clamp, M. & Barton, G. J. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics25, 1189–1191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hughes, C. S. et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc.14, 68–85 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Batth, T. S. et al. Protein aggregation capture on microparticles enables multipurpose proteomics sample preparation. Mol. Cell. Proteomics18, 1027–1035 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol.30, 918–920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendes, M. L. et al. An integrated workflow for crosslinking mass spectrometry. Mol. Syst. Biol.15, e8994 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Combe, C. W., Graham, M., Kolbowski, L., Fischer, L. & Rappsilber, J. xiVIEW: visualisation of crosslinking mass spectrometry data. J. Mol. Biol.436, 168656 (2024). [DOI] [PubMed] [Google Scholar]
- 80.Schiffrin, B., Radford, S. E., Brockwell, D. J. & Calabrese, A. N. PyXlinkViewer: a flexible tool for visualization of protein chemical crosslinking data within the PyMOL Molecular Graphics System. Protein Sci.29, 1851–1857 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RECQL5 and the TCR complex coordinate to regulate the transcription elongation rate.
Unprocessed gels.
Unprocessed gels.
Unprocessed gels.
Unprocessed gels and western blots.
Unprocessed gels.
Unprocessed gels.
Unprocessed gels.
Statistical source data.
Unprocessed gels and western blots.
Data Availability Statement
The cryo-EM reconstructions and final models were deposited in the EMDB under accession code EMD-52440 and the PDB under accession code 9HVO for the EC–RECQL5 complex, EMD-52443 (overall), EMD-52441 (SPT6 stalk), EMD-52442 (PAF) and PDB 9HVQ for the EC*–RECQL5 complex, and EMD-52449 and PDB 9HWG for the EC–TCR–RECQL5 complex. The raw data for cross-linking-coupled mass spectrometry of the EC*–RECQL5 complex are available at PRIDE PXD062042. Source data are provided with this paper.