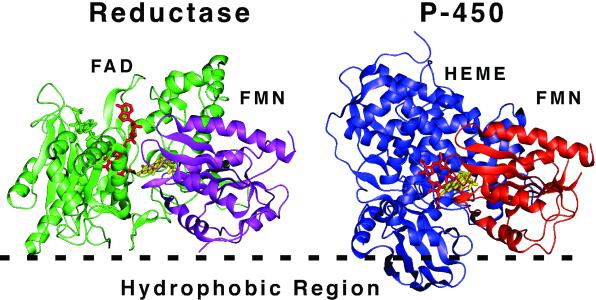
Figure 8.
Possible orientation of redox transfer complexes on the membrane surface. Cytochrome P450 reductase is shown based on the probable orientation of the reductase at a membrane surface (hydrophobic region). Cytochrome P450 was oriented such that the FMN domain (orange) of the known structure of bacterial cytochrome P450 CYP102 is roughly superimposable on the FMN domain of P450 reductase (purple). The resulting orientation of cytochrome P450 with respect to the membrane corresponds to Model 1 of Fig. 6. Note that whereas the FMN (yellow) of reductase accepts electrons from FAD (green), in the P450 structure the FMN (yellow) is in close proximity to the heme cofactor (red). The structures of reductase (PDB ID 1AMO) and CYP102 (PDB ID 1BVY) were obtained from the Protein Data Bank, www.rcsb.org.