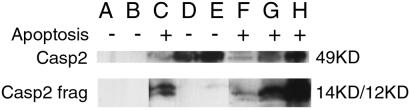
Figure 5.
Mutant Caspase 2s that do not interact with cyclin D3 are expressed at different levels, are not cleaved when expressed in HEK 293 cells, but are cleaved when coexpressed with wt Caspase 2. Immunoprecipitation Western probed with anti-Myc monoclonal antibody showing patterns of cleavage for mutant Caspase 2 in HEK 293 cells transfected with control Myc-fusion vector pcDNA3.1 and HA fusion vector pBC147 (lane A); pcDNA3.1 and pBC147-wt-Caspase 2 (lane B); pcDNA3.1-wt-Caspase 2 + pBC147 (lane C); pcDNA3.1-Y175N-Caspase 2 + pBC147 (lane D); pcDNA 3.1.-A242P-Caspase 2 + pBC147 (lane E); pcDNA3.1-wt-Caspase 2 + pBC147-wt-Caspase 2 (lane F); pcDNA3.1-Y175N-Caspase 2 + pBC147-wt-Caspase 2 (lane G); or pcDNA3.1-A242P-Caspase 2 + pBC-147-wt-Caspase 2 (lane H). + indicates that morphological evidence of apoptosis is present in the respective cell cultures; − indicates no evidence of apoptosis. The uncleaved Caspase 2 band runs at 49 kDa, as visualized in lanes D and E, in which the mutant Caspase 2 was coexpressed with a vector control. The Caspase 2 14 kDa and 12 kDa cleavage products are seen in lanes C (wt), F (wt), G (Y175N), and H (A242P). These bands correlate with the coexpression.