Abstract
Introduction
Ofatumumab demonstrated superior efficacy and similar safety versus teriflunomide in ASCLEPIOS I/II in people with relapsing multiple sclerosis; no new safety concerns and sustained efficacy were observed up to 4 years in the open-label extension study ALITHIOS. Here, we further characterise the safety and efficacy of ofatumumab up to 5 years by discussing infection outcomes in the COVID-19 era and providing a comprehensive overview of participant disability outcomes.
Methods
Safety (N = 1969; participants who received ≥ 1 dose of ofatumumab in ASCLEPIOS I/II, APLIOS, APOLITOS, or ALITHIOS) and efficacy sets (N = 1882; participants randomised to ofatumumab [OMB-OMB] or teriflunomide [TER-OMB] in ASCLEPIOS I/II, regardless of whether they entered ALITHIOS) were analysed. Data cutoff: 25 September 2022.
Results
The exposure-adjusted incidence rates (per 100 patient-years) of adverse events (AEs, 124.65), serious AEs (4.68), serious infections (1.63), and malignancies (0.32) remained consistent with previous findings up to 5 years of follow-up, with no new safety signals identified. With ofatumumab treatment up to 5 years, > 80% of patients remained free of 6-month confirmed disability worsening (6mCDW). Annualised relapse rates (ARR) remained low, and magnetic resonance imaging (MRI) activity was almost completely suppressed with OMB-OMB through years 1–5; after switching from teriflunomide (years 2–3), pronounced reductions in ARR/MRI activity were observed with low rates sustained through years 3–5. During year 5, 9 of 10 participants in both groups were free of disease activity (NEDA-3).
Conclusion
Ofatumumab has a favourable benefit-risk profile that is sustained up to 5 years.
Trial registration: ALITHIOS (NCT03650114): https://clinicaltrials.gov/ct2/show/NCT03650114
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-025-00784-0.
Keywords: Disability, High-efficacy therapies, Ofatumumab, Relapsing multiple sclerosis, Safety
Key Summary Points
| Why carry out this study? |
| The Phase 3 ASCLEPIOS I and II trials showed the superiority of ofatumumab over teriflunomide in reducing annualised relapse rate, magnetic resonance imaging (MRI) lesion activity, and delaying disability worsening, while maintaining a manageable safety profile for up to 2.5 years. |
| Long-term assessment of the safety and efficacy of B-cell depleting therapies, such as ofatumumab, is crucial to better inform the care of people with relapsing multiple sclerosis (RMS). |
| The open-label extension ALITHIOS study is aimed at assessing the long-term safety and efficacy of ofatumumab, including comprehensive results on patient outcomes related to disability. |
| What was learned from the study? |
| The safety profile of ofatumumab was consistent with previous findings and was sustained for up to 5 years in patients with RMS, with no new safety signals identified, reinforcing the long-term safety profile of ofatumumab. |
| Continuous ofatumumab treatment (i.e., early initiation of ofatumumab) for up to 5 years showed sustained efficacy in relapse rate reduction, profound suppression of MRI lesion activity, high rates of ‘no evidence of disease activity’ (NEDA-3), and reductions in long-term disability accrual. Specifically, results suggested an efficacy benefit of early use of ofatumumab on disability outcomes that was not recovered in those initially randomised to teriflunomide and who later switched to ofatumumab. |
| Overall, the results presented here reinforce the favourable, long-term (5-year) benefit–risk profile of ofatumumab, supporting its early initiation in patients with RMS. |
Introduction
Ofatumumab is a fully human anti-CD20 monoclonal antibody (mAb) approved for relapsing multiple sclerosis (RMS) in adults in over 90 countries [1–3]. More than 100,000 patients with RMS have been treated with ofatumumab worldwide since its launch in 2020 (223,001 patient-years [PYs]) [3, 4]. Ofatumumab has a high affinity and potency for a distinct CD20 epitope on B cells, resulting in marked B-cell depletion at a lower concentration than that required with other anti-CD20 mAbs [5]. A small population of CD20-expressing T cells are also depleted by ofatumumab [1, 5]. Ofatumumab's 20 mg monthly dose regimen (after the initial loading doses) provides rapid and sustained depletion of B cells, which can be seen as early as 2 weeks after treatment initiation [5–7]. Ofatumumab is a subcutaneous (SC) anti-CD20 mAb intended for self-administration at home (following training by a healthcare professional) with no premedication required [1, 2, 5].
In observational settings, early initiation of high-efficacy therapies improved long-term outcomes for patients with RMS compared with initiating on a lower efficacy therapy and later switching to a higher efficacy therapy [8, 9]. The phase 3 ASCLEPIOS I and II trials in patients with RMS demonstrated the superiority of ofatumumab (20 mg SC) compared with teriflunomide in reducing the annualised relapse rate (ARR), suppressing magnetic resonance imaging (MRI) lesion activity and delaying worsening of disability while maintaining a manageable safety profile for up to ~ 2.5 years of ofatumumab treatment [10]. These results were consistent, with equivalent or greater effect sizes, in a subgroup of recently diagnosed and treatment-naive patients, supporting the use of ofatumumab as a first-line therapy for patients with RMS [11].
Assessment of long-term safety and efficacy is important to extend understanding of the benefit-risk profile of ofatumumab. Previously, sustained efficacy and a favourable benefit–risk safety profile were demonstrated with ofatumumab treatment for up to 4 years in patients with RMS [12]. Here, we expand upon the 4-year data by not only describing the safety and efficacy of ofatumumab up to 5 years, but by discussing infection outcomes in the COVID-19 era and presenting comprehensive analyses on patient outcomes related to disability, including progression independent of relapse (PIRA) and confirmed disability improvement (CDI).
Patient and Methods
Trial Design and Patients
The design and methodology of ASCLEPIOS I/II, APLIOS, and APOLITOS were previously reported in detail [10, 13, 14]. ALITHIOS is an ongoing, phase 3b, open-label, umbrella extension study designed to evaluate the long-term safety and efficacy of ofatumumab (20 mg SC every 4 weeks) in patients with RMS [12, 15, 16]. Patients who completed treatment in the core periods of ASCLEPIOS I/II, APLIOS, or APOLITOS could enter ALITHIOS (see Figure S1). For key inclusion/exclusion criteria, refer to Supplementary Materials. ALITHIOS (initiated 22 November 2018) has an estimated completion date of 30 December 2027 [15]. Interim analyses presented here are from the core and extension periods (data cutoff: 25 September 2022) [15]. The trial is registered with ClinicalTrials.gov (NCT03650114).
These clinical studies were designed and implemented, executed, and reported in accordance with the International Conference on Harmonisation Tripartite guidelines for Good Clinical Practice [17] with applicable local regulations (including European Directive 2001/20/EC, US CFR 21), and with the ethical principles laid down in the Declaration of Helsinki [18]. The protocol was approved by an institutional review board or ethics committee at each trial site. All the patients or their legal representatives provided written informed consent before commencing trial-related procedures.
Analysis populations
Details regarding the definitions of different analysis populations can be found in the Supplementary Materials section.
Analysis of Early Versus Delayed Initiation of Ofatumumab
Safety analyses included patients who received at least one dose of ofatumumab in ASCLEPIOS I/II, APLIOS, APOLITOS or ALITHIOS, including those who completed or discontinued ofatumumab during these trials: within the safety analyses, the continuous ofatumumab group includes those who received the first dose of ofatumumab in ASCLEPIOS I/II, APLIOS, or APOLITOS, and the newly switched ofatumumab group includes those who were randomised to teriflunomide in ASCLEPIOS I/II and were switched to ofatumumab in ALITHIOS. For efficacy analyses, the OMB-OMB group included patients randomised to receive ofatumumab in ASCLEPIOS I/II, and the TER-OMB group consisted of patients randomised to receive teriflunomide in ASCLEPIOS I/II, regardless of whether or not they entered ALITHIOS (see Figure S1). For further details, see Supplementary Materials.
Safety and Tolerability Evaluation
Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events version 5.0 [19], with preferred terms per Medical Dictionary for Regulatory Activities version 25.1. An independent expert reviewed cases of opportunistic infection. For further details on safety outcomes and compliance data, see Supplementary Materials.
Efficacy Endpoints
The efficacy endpoints evaluated in this study include confirmed disability worsening (CDW) events (confirmed increase in Expanded Disability Status Scale (EDSS) score for ≥ 3 or ≥ 6 months [3mCDW or 6mCDW]), 6-month confirmed PIRA (6mPIRA), 6-month confirmed relapse-associated worsening (6mRAW), and 6-month CDI (6mCDI). In addition, ARR, gadolinium-enhancing (Gd+) T1 lesions per scan, new/enlarging T2 (neT2) lesions per year, and no evidence of disease activity (NEDA-3) status (absence of 6mCDW events, confirmed relapses and MRI activity) were analysed in the core and extension periods, yearly, and overall. Serum neurofilament light chain (sNfL) concentration, annualised brain volume change (ABVC) in the core and extension periods, and percentage brain volume change (PBVC) by year were also assessed. For further details, see Supplementary Materials.
Statistical Analyses
The exposure-adjusted incidence rates (EAIR) of AEs and serious AEs (SAEs) were provided, where time-at-risk was from the first dose of ofatumumab to 100 days after the last dose of ofatumumab for patients without the event. The annualised rate of serious infections was estimated using negative binomial regression where multiple events of a patient were considered. The rate of serious COVID-19 per calendar year was estimated using Poisson regression. Cumulative 3/6mCDW, 6mPIRA, 6mRAW, and 6mCDI event rates were assessed using Kaplan–Meier curves. The yearly ARR, by-year estimates of Gd+ T1 lesions per scan, and annualised rates of neT2 lesions were estimated from negative binomial regression. NEDA-3 was analysed annually using logistic regression. sNfL concentration and PBVC estimates were assessed by mixed-effect modelling of repeated measures. ABVC estimates were obtained from a random coefficient model. All p values across different outcomes are nominal, and p < 0.05 was considered nominally statistically significant. For further details on statistical analyses, see Supplementary Materials.
Results
Patients
Safety Analyses
In total, 1969 patients were included in the safety analysis set. Core study baseline disease characteristics of the continuous ofatumumab (n = 1292) and newly switched groups (n = 677) were comparable (see Table S1). Of 1969 patients in the safety analysis set, 1703 (86.5%) completed the core studies and entered ALITHIOS. As of data cutoff, the total ofatumumab exposure was 5185.3 PYs; continuous ofatumumab group: 3340.4 PYs; newly switched group: 1844.9 PYs; the median ofatumumab exposure was 4.4 years in the continuous ofatumumab group and 3.0 years in the newly switched group. The overall mean compliance with ofatumumab treatment was 96.0% (see Supplementary Materials: Endpoints: definitions and assessment criteria and Table S2). At data cutoff, 406 patients had completed 5 years of ofatumumab treatment (see Table S3).
Efficacy Analyses
A total of 1882 patients were randomised to treatment in ASCLEPIOS I/II (OMB: 946; TER: 936). Of these, 1367 (72.6%) entered ALITHIOS (OMB-OMB: n = 690; TER-OMB: n = 677; see Figure S1). At data cutoff, 1145 of 1367 (83.8%) were receiving ofatumumab. Demographics and disease characteristics were generally well balanced between groups at core study baseline (see Table S4). In ALITHIOS, the most common reasons for study discontinuation were patient/guardian decision (6.6%) and AEs (4.5%).
Safety
AE Profile
Based on data with up to 5 years of follow-up, safety was consistent with previous reports from the core studies and 4-year follow-up data from ALITHIOS [10, 12, 16]; no new safety signals emerged. Ofatumumab safety is summarised in Table 1. Of 1969 patients in the safety analysis set, 1771 (89.9%) had ≥ 1 AE; the exposure-adjusted incidence rate was 124.65 per 100 patient-years [PYs] (95% confidence interval [CI]: 118.97–130.59); the EAIR of the ASCLEPIOS I/II core period was 188.55 (95% CI: 175.86–202.16). The most common AEs were infections (COVID-19 [30.3%], nasopharyngitis [19%], upper respiratory tract infections [12.8%], and urinary tract infections [12.7%]). Most (90.3%) infections resolved without discontinuing ofatumumab. The proportion of participants who discontinued ofatumumab due to AEs was 7.1%. SAEs occurred in 289 of 1969 patients (14.7%); the EAIR was 4.68 SAEs per 100 PYs (95% CI: 4.17–5.26).
Table 1.
Safety summary (safety analysis set)
| Adverse event | ASCLEPIOS I/II core period, ofatumumab group (N = 946)a | Overall ofatumumab (N = 1969)b | ||
|---|---|---|---|---|
| n (%) | EAIR (95% CI) | n (%) | EAIR (95% CI) | |
| Patients with at least one AE | 791 (83.61) | 188.55 (175.86–202.16) | 1771 (89.90) | 124.65 (118.97–130.59) |
| Patients with at least one SAE | 83 (8.77) | 5.56 (4.48–6.89) | 289 (14.70) | 4.68 (4.17–5.26) |
| AEs leading to ofatumumab discontinuation | 54 (5.70) | – | 139c (7.10) | – |
| Infections and infestations | 488 (51.58) | 51.14 (46.80–55.88) | 1334 (67.75) | 40.99 (38.85–43.25) |
| Serious infections | 24 (2.54) | 1.55 (1.04–2.31) | 106 (5.38) | 1.63 (1.35–1.97) |
| Serious infections (excluding COVID-19) | 24 (2.54) | 1.55 (1.04–2.31) | 61 (3.09) | 0.93 (1.35–1.20) |
| Serious COVID-19 infections | 0 | 0 | 50 (2.53) | 0.75 (0.57–1.00) |
| Injection-related systemic reactions | 195 (20.61) | 15.49 (13.46–17.83) | 508 (25.79) | 10.06 (9.22–10.98) |
| Injection site reactions | 103 (10.88) | 7.21 (5.94–8.74) | 243 (12.3) | 4.08 (3.60–4.63) |
| Malignancies | 5 (0.53) | 0.32 (0.13–0.77) | 21 (1.06) | 0.32 (0.21–0.48) |
| Deaths | 0 | - | 9d (0.46) | - |
Preferred terms are according to MedDRA version 25.1. Data are from the safety analysis set
AE adverse event; CI confidence interval; EAIR exposure-adjusted incidence rate (per 100 patient-years); Ig immunoglobulin; SAE serious adverse event
aData are from the core period
bData from both the core and open-label extension periods
cAEs related to reduced IgM levels are the most common reason for treatment discontinuation (n = 71 [3.6%])
dCauses of death were sudden death (n = 1); suicide (n = 1); COVID-19 and COVID-19 pneumonia (n = 2); COVID-19 (n = 2); intestinal metastasis (n = 1); pneumonia and septic shock (n = 1; unrelated to study drug; had medical history of kyphosis; treatment was discontinued due to pneumonia and septic shock]), and pneumothorax (n = 1; developed during COVID-19 infection, and in autopsy the primary cause of death is COVID-19 pneumonia)
Safety findings for 406 patients who completed 5 years of ofatumumab treatment are summarised in Table S3. Among these 406 patients, 389 (95.8%) had ≥ 1 AE; the EAIR was 122.72 AEs per 100 PYs (95% CI: 111.12–135.55), and the most common AEs were the same as those reported for the overall safety population. SAEs were reported in 73 of these 406 patients (18.0%); the EAIR was 3.88 SAEs per 100 PYs (95% CI: 3.09–4.89) (see Table S3). No cases of progressive multifocal leukoencephalopathy were reported.
Serious Infections
The EAIR for serious infections was 1.63 per 100 PYs (95% CI: 1.35–1.97), which is consistent with the rate in the ASCLEPIOS I/II trials (EAIR: 1.55) (see Table 1). The most common serious infections included COVID-19 pneumonia (1.37%), COVID-19 (1.37%) and appendicitis (0.7%). Most serious infections (56.6%) were grade 3 in severity, with 34.0% being grade 1/2 and 9.4% being grade 4, and most (92.6%) resolved without discontinuing ofatumumab treatment. Among patients who completed 5 years of treatment, the EAIR for serious infections was 1.28 per 100 PY (95% CI: 0.87–1.89; see Table S3), and the most common serious infections were consistent with those reported for the overall safety population.
The annualised rates (95% CI) of serious infections (excluding COVID-19) were low and stable throughout 5 years of ofatumumab treatment (year 1: 0.013 [0.008–0.020]; year 2: 0.010 [0.006–0.017]; year 3: 0.011 [0.006–0.018]; year 4: 0.008 [0.004–0.017]; year 5: 0.006 [0.002–0.019]; see Figure S2A). From 2020 to 2023, the annualised rate of serious COVID-19 cases remained low (in 2020: 0.004 [0.002–0.009]), with the highest rate observed in 2021, during the COVID-19 pandemic (0.020 [0.014–0.028]), and this number decreasing through to the data cutoff point (25 September 2022) (0.012 [0.007–0.021]; see Figure S2B).
Malignancies
The overall incidence of malignancies was 1.06% (n = 21), with an EAIR of 0.32 per 100 PYs (95% CI: 0.21–0.48). The EAIRs for malignancies did not increase over time (year 1: 0.47 [95% CI: 0.25–0.91]; year 5: 0.37 [95% CI: 0.09–1.50]; see Figure S3). In patients reporting malignancies (irrespective of type), the median onset since the first dose of ofatumumab was 1.5 years (range 0.5–4.8 years). Among the 406 patients who completed 5 years of treatment, one had a malignancy (EAIR, 0.05 per 100 PYs) (Table S3).
Deaths
No deaths occurred in ASCLEPIOS I/II, and in total, nine deaths occurred (0.46%), all during the extension period (see Table 1). All were reported by the investigators as being unrelated to ofatumumab. Causes of deaths were sudden death (n = 1), suicide (n = 1), COVID-19/COVID-19 pneumonia (n = 4, all during the COVID-19 pandemic; n = 3 patients were unvaccinated, while n = 1 received two doses of vaccine), intestinal metastasis (n = 1), pneumonia and septic shock (n = 1; patient had a medical history of kyphosis) and pneumothorax (n = 1).
IgG and IgM
Mean serum IgG levels remained stable from the first dose of ofatumumab for up to 5 years (see Figure S4A). The IgG levels remained above the lower limit of normal (LLN) (5.65 g/L) in 98% of patients at all visits, and the proportion of patients with IgG levels below the LLN remained consistent for up to 5 years of ofatumumab treatment (see Figure S4B). The mean IgM levels initially declined but then stabilised and remained above LLN (0.40 g/L) from the first dose of ofatumumab for up to 5 years. IgM levels remained above LLN at all assessments in 69.4% of patients (see Figure S4D). The proportion of patients reaching IgM levels below the LLN was 10.6% after 1 year of ofatumumab treatment and 24.1% with up to 5 years of treatment (see Figure S4E). Over 5 years, the proportion of participants who interrupted ofatumumab treatment due to low IgG or IgM was 0.2% and 10.3%, respectively. Treatment discontinuations due to low IgG or IgM were reported in 0.2% and 3.6% of the overall population, respectively. Sensitivity analyses showed no major difference in the overall mean IgG/IgM trends after imputing IgG/IgM levels over time for patients who interrupted ofatumumab due to either low IgG or IgM levels (see Figures S4C and S3F). Serious infections were reported in 3 of 40 patients (7.5%) with IgG levels below the LLN (compared with 99 of 1926 [5.1%] in patients with IgG levels above LLN) and 10 of 601 patients (1.7%) with IgM levels below the LLN (compared with 72 of 1365 [5.3%] in patients with IgM levels above LLN); all had resolved, with only one serious infection in a patient with IgM below the LLN, which led to treatment discontinuation. See Supplementary Materials and Table S5 for more information.
Lymphocyte and Neutrophil Levels
The mean lymphocyte and neutrophil levels remained stable and were above the LLN with up to 5 years of treatment with ofatumumab (see Supplementary Materials and Figure S5).
For further information on safety outcomes, see Supplementary Materials.
Efficacy assessments
6-Month CDW and 6-Month CDI Events
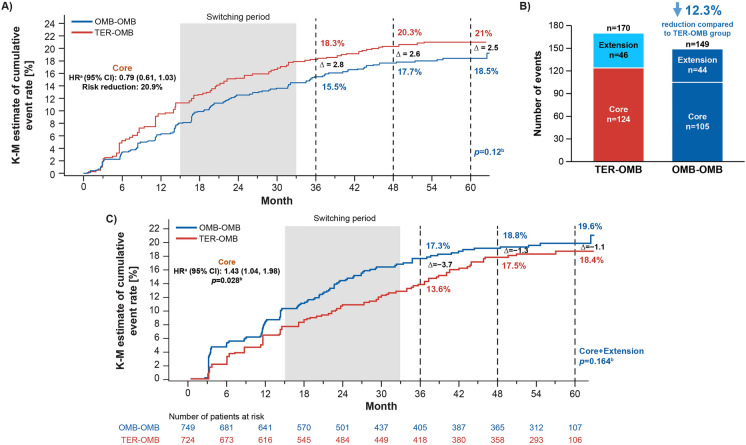
With up to 5 years of ofatumumab treatment, > 80% of patients remained free of 6mCDW. Cumulative 6mCDW event rates (Kaplan–Meier estimate) remained numerically lower in the OMB-OMB versus TER-OMB group (6mCDW rate at Month 60: 18.5% [95% CI: 15.9–21.4] vs 21% [95% CI: 18.3–24.0; overall log-rank p = 0.12; see Fig. 1A). Cumulative numbers of 6mCDW events were numerically lower in the OMB-OMB versus TER-OMB group (6mCDW events: n = 149 vs n = 170, 12.3% lower for OMB-OMB vs TER-OMB [see Fig. 1B]). Similar patterns were identified for 3mCDW (see Figure S6).
Fig. 1.
6-month confirmed disability worsening (6mCDW): A Kaplan–Meier estimates of cumulative 6mCDW event rates; B cumulative number of 6mCDW events (efficacy analysis set); C 6-month confirmed disability improvement (6mCDI): Kaplan–Meier estimates of cumulative 6mCDI event rates (efficacy analysis set). Data are from the efficacy analysis set. Superior efficacy of ofatumumab over teriflunomide in the core period was established previously (for more information, refer to Hauser et al. [9]). Cutoff for the core and open-label extension periods was based on the first dose of ofatumumab in the open-label extension period. ‘∆ (delta symbol)’ refers to the difference in KM estimates (TER-OMB group minus OMB-OMB group). Switching period starts at the minimum switch-day and ends at the maximum switch-day among subjects in the TER-OMB group, where for each subject, switch-day is the day of the first dose of ofatumumab relative to treatment start date of the core studies. Patients with baseline total EDSS scores of 0, 1, and 1.5 are excluded from analyses as no disability improvement is possible for these patients. aHR determined by Cox regression model. bp value is a log-rank test. 6mCDI 6-month confirmed disability improvement; 6mCDW 6-month confirmed disability worsening; CI confidence interval; EDSS Expanded Disability Status Scale; HR hazard ratio; KM Kaplan–Meier; n number of patients; OMB-OMB continuous ofatumumab; TER-OMB switch from teriflunomide to ofatumumab
At Month 60, the cumulative event rate (Kaplan–Meier estimate) for 6mCDI was numerically higher for the OMB-OMB group (19.6% [95% CI: 16.6–22.9]) versus the TER-OMB group (18.4% [95% CI: 15.4–21.9]; see Fig. 1C). In the core period, the chances of 6mCDI were 44% higher for patients in the OMB-OMB group versus those in the TER-OMB group (hazard ratio [HR] 1.44 [95% CI: 1.04–1.98]; p = 0.028); the HR for 6mCDI in the extension period, when all patients were receiving ofatumumab, was 0.77 (95% CI: 0.49–1.20; p = 0.242) for the OMB-OMB group versus the TER-OMB group.
6mPIRA and 6mRAW Events
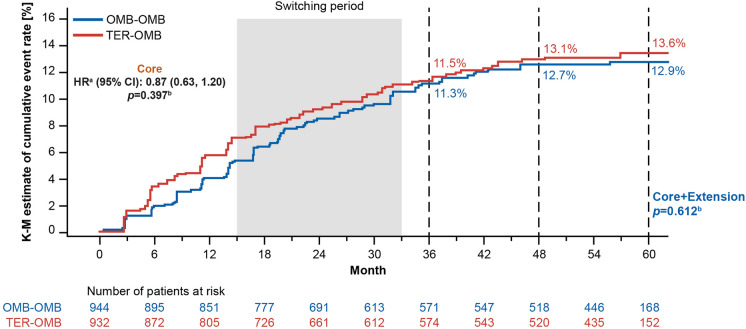
The main contributor to overall 6mCDW was 6mPIRA in both the OMB-OMB and TER-OMB groups (69.8% and 63.5% of 6mCDW events, respectively, see Figure S7). Overall, 6mPIRA events were experienced in 12.9% (95% CI: 10.7–15.5]) of the OMB-OMB group versus 13.6% [95% CI: 11.3–16.2] of the TER-OMB group (see Fig. 2). Low levels of 6mRAW were observed in both the OMB-OMB and TER-OMB groups, with a flattening of the RAW curve in the extension period for the TER-OMB group following the switch to OMB (see Figure S8); 6mRAW contributed to 26.8% of 6mCDW in the OMB-OMB group and 24.7% of 6mCDW in the TER-OMB group (see Figure S7).
Fig. 2.
6-month confirmed progression independent of relapse activity (6mPIRA): Kaplan–Meier estimates of cumulative 6mPIRA event rates (efficacy analysis set). Data are from the efficacy analysis set. Switching period starts at the minimum switch-day and ends at the maximum switch-day among subjects in the TER-OMB group, where for each subject, switch-day is the day of the first dose of ofatumumab relative to the treatment start date of the core studies. aHR determined by Cox regression model. bp value is a log-rank test. 6mPIRA 6-month confirmed progression independent of relapse activity; HR hazard ratio; KM Kaplan–Meier; OMB-OMB continuous ofatumumab; TER-OMB switch from teriflunomide to ofatumumab
Relapses
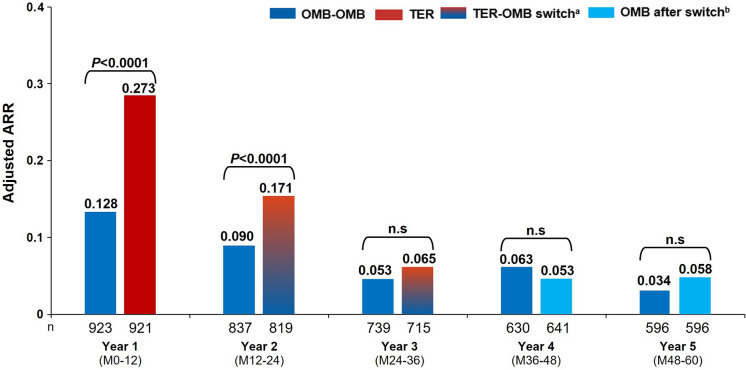
A sustained low ARR was observed in the OMB-OMB group with up to 5 years of treatment, from 0.128 at year 1 to 0.034 through year 5 (see Fig. 3). Switch from teriflunomide to ofatumumab resulted in a pronounced reduction in ARR from year 2 to 3 (0.171 to 0.065), and a sustained low ARR was observed onwards through year 5 (0.058; see Fig. 3). See Supplementary Materials: Additional efficacy assessments and Figures S9A and S9B for additional within- and between-group analyses (ARR in the core vs extension periods). Earlier (OMB-OMB) versus later (TER-OMB) initiation of ofatumumab was associated with a 40% reduction in the cumulative number of confirmed relapses over the core plus extension periods (see Figure S9C).
Fig. 3.
Annualised relapse rate (ARR) in the core and extension periods by year (year 1–5) (efficacy analysis set). Patient numbers at corresponding years for ARR may differ due to missing covariates. ARR was estimated from a negative binomial model with a log link adjusted for treatment, visit, region, number of relapses in previous year, baseline EDSS, baseline number of Gd+ lesions, age at baseline, and a treatment by visit interaction. Log-transformed exposure time (in years) per visit is included as an offset variable to annualise the relapse rate at each visit. Baseline variables are from the core study baseline. aTER-OMB switch: patients transitioning from TER to OMB; due to event-driven core study design (flexible duration), patients transitioned at various exposure time points; i.e., the switch from TER to OMB started from year 2 and was completed by year 3. bOMB after switch: patients who switched from teriflunomide to ofatumumab. ARR annualised relapse rate; EDSS Expanded Disability Status Scale; Gd+ gadolinium-enhancing; M month; n.s. non-significant; OMB ofatumumab; OMB-OMB continuous ofatumumab; TER teriflunomide; TER-OMB switch from teriflunomide to ofatumumab
MRI Assessments
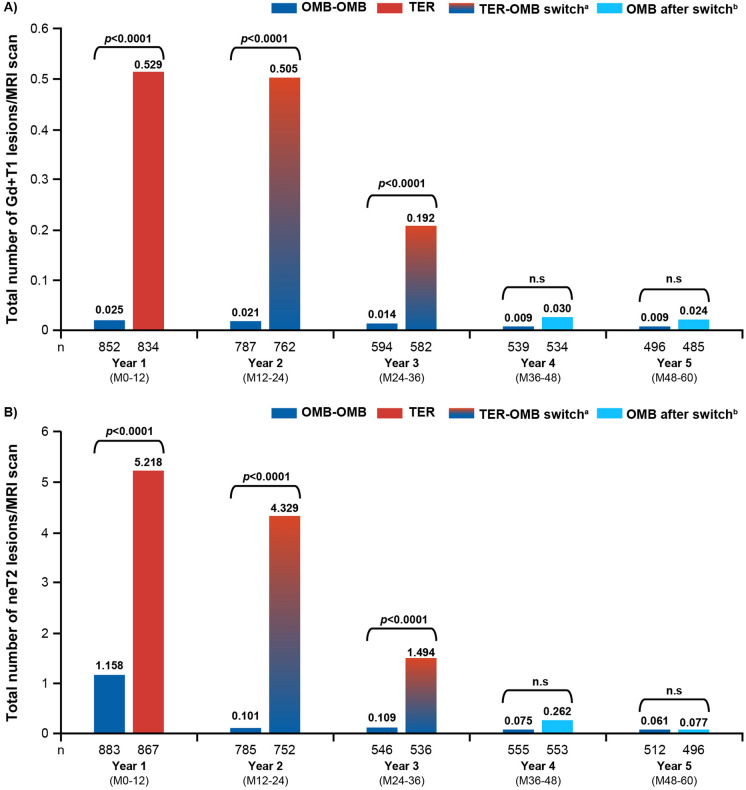
Participants in the OMB-OMB group maintained a profound suppression of the number of Gd+ T1 lesions per scan from year 1 (0.025) through year 5 (0.009). Participants in the TER-OMB group showed a rapid suppression of Gd+ T1 lesions from year 2 (0.505) to year 3 (0.192), to closely match the OMB-OMB group through year 5 (0.024; see Fig. 4A). Similarly, a profound suppression of the mean number of neT2 lesions was maintained with OMB-OMB from year 1 (1.158) through year 5 (0.061), whereas participants in the TER-OMB group showed suppression of neT2 lesions from year 2 (4.329) to year 3 (1.494), to closely match the OMB-OMB group through year 5 (0.077; see Fig. 4B). See Supplementary Materials: Additional efficacy assessments and Figures S10A, S10B, S11A, and S11B for additional within- and between-group analyses (MRI lesion activity in the core vs extension periods). Earlier (OMB-OMB) versus later (TER-OMB) initiation of ofatumumab was associated with a 94% and 83% reduction in the cumulative number of Gd+ T1 and neT2 lesions, respectively, over the core plus extension periods (see Figure S10C and S11C).
Fig. 4.
Lesion activity on MRI in the core and extension periods: A mean number of Gd+ T1 lesions per scan by year; B comparison of mean numbers of annualised neT2 lesions by year (efficacy analysis set). Data are from the efficacy analysis set. Patient numbers at corresponding years for Gd+ T1 lesions and neT2 lesions may differ due to missing covariates and post-baseline MRI visits. Results are obtained from a negative binomial model on treatment, visit, baseline number of Gd+ T1 lesions (A) or T2 lesions (B), age at baseline, and a treatment by visit interaction. An unstructured within-subject correlation was assumed. The natural log of number of assessments per year is used as offset to annualise the lesion rate at each visit. Baseline variables are from the core study baseline. aTER-OMB switch: patients switching from TER to OMB; due to event-driven core study design (flexible duration), patients switched at various exposure time points; i.e., the switch from TER to OMB started from year 2 and was completed by year 3. bOMB after switch: patients who switched from teriflunomide to ofatumumab. Gd+ gadolinium-enhancing; neT2 new or enlarging T2; OMB ofatumumab; OMB-OMB continuous ofatumumab; M month; MRI magnetic resonance imaging; TER teriflunomide; TER-OMB switch from teriflunomide to ofatumumab
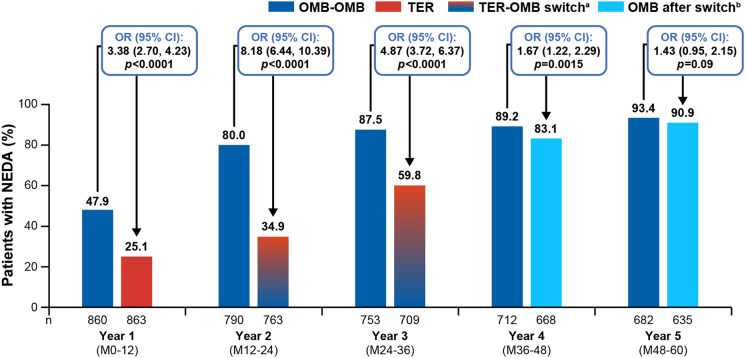
Annual NEDA-3 Status
In line with the rapid and sustained depletion of B cells induced by ofatumumab (see Supplementary Materials), the proportion of patients achieving NEDA-3 in the OMB-OMB group increased rapidly from 47.9% in year 1 to 80% in year 2, reaching 93.4% in year 5. Evidence that ofatumumab rapidly controls disease activity after switch from teriflunomide is provided by the rapid depletion of B cells (see Supplementary Materials) and also by the rise in NEDA-3 rates in the TER-OMB group from 25.1% at year 1 to 34.9% during year 2 as the switching period began and increased to a greater extent during year 3 (59.8%) as the number of patients switched to ofatumumab increased further. During year 4, after all patients had been switched to ofatumumab, 83.1% in the TER-OMB group had achieved NEDA-3 and, by year 5, NEDA-3 was achieved by 9 of 10 patients in both the OMB-OMB and TER-OMB groups (see Fig. 5). Regarding the individual components of NEDA-3, a numerically greater percentage of patients in the OMB-OMB versus TER-OMB group were free of 6mCDW (82.9% vs 80.7%), Gd+ T1 lesions (97.1% vs 64.6%), new/enlarging T2 lesions (47.7% vs 25.1%) and confirmed relapses (75.6% vs 63.2%) over 5 years. See Table 2 for data on each component of NEDA-3.
Fig. 5.
No evidence of disease activity (NEDA-3): Annual NEDA-3 status for up to 5 years of ofatumumab treatment (efficacy analysis set). Data are from the modified efficacy analysis set for NEDA-3. All p values are nominal. The statistical model used logistic regression to adjust for treatment and region as factors and for age, baseline EDSS score, and number of Gd+ T1 lesions at baseline as covariates. aTER-OMB switch: patients transitioning from TER to OMB; due to event-driven core study design (flexible duration), patients transitioned at various exposure time points; i.e., the switch from TER to OMB started from year 2 and was completed by year 3. bOMB after switch: patients who switched from teriflunomide to ofatumumab. CI confidence interval; Gd+ gadolinium-enhancing; N total number of patients in each group excluding those who discontinued early for reasons other than lack of efficacy or death and had NEDA before early discontinuation; NEDA-3 three-parameter no evidence of disease activity; M month; OMB-OMB continuous ofatumumab; OR odds ratio; TER-OMB switch from teriflunomide to ofatumumab
Table 2.
Proportion of patients free of individual NEDA-3 components over 5 years (modified efficacy analysis set)
| NEDA-3 components | OMB-OMB N = 843 n/M (%) |
TER-OMBa N = 855 n/M (%) |
|
|---|---|---|---|
| Patients free of Gd+ T1 lesions | 796/820 (97.1) | 542/839 (64.6) | |
| Patients free of neT2 lesions | 391/820 (47.7) | 211/839 (25.1) | |
| Patients free of confirmed relapses | 620/820 (75.6) | 530/839 (63.2) | |
| Patients free of 6mCDW | 680/820 (82.9) | 677/839 (80.7) | |
Data are from the modified efficacy analysis set for NEDA-3
6mCDW 6-month confirmed disability worsening; Gd+ gadolinium-enhancing; NEDA-3 three parameter no evidence of disease activity; neT2 new or enlarging T2; OMB-OMB continuous ofatumumab; TER-OMB switch from teriflunomide to ofatumumab
aPatients who completed treatment with teriflunomide in the core period were switched to ofatumumab in the open-label extension period
Whole-Brain Volume
In the OMB-OMB group, percentage brain volume change (PBVC) by year remained low at < 1.5% with up to 5 years of treatment, equivalent to approximately 0.3% PBVC on average per year (PBVC at year 5 for OMB-OMB vs TER-OMB was −1.42% vs −1.66%, respectively, p = 0.002; see Figure S12). The annualized brain volume change (ABVC), relative to core baseline, was lower in the OMB-OMB group than in the TER-OMB group in the core period (−0.34% vs −0.42%; p = 0.115) and was similar in both groups in the extension periods (−0.27% vs −0.28%; p = 0.666; see Supplementary materials [see Table S6] for more information).
sNfL Concentrations
In the OMB-OMB group, sNfL levels (pg/mL) were lower than TER-OMB (Month 3: 9.61 vs 10.34; Month 12: 8.04 vs 10.20; and Month 24: 8.19 vs 10.10; all p < 0.001; Month 36: 8.38 vs 8.91, p = 0.011) and remained low for up to 5 years (Month 60: 8.67; see Figure S13A). Switching from teriflunomide to ofatumumab resulted in a reduction in sNfL levels: in the TER-OMB group, sNfL levels remained higher than OMB-OMB group up to 6 months following the switch (9.07 vs 8.30), and after this time point, sNfL levels were low in both groups (Month 36 of extension: 8.68 vs 8.45; Month 48: 8.59 vs 8.50; and Month 60: 8.63 vs 9.12) (see Figure S13B).
Discussion
This 5-year analysis provides additional insights into safety, longer-term disability-related and additional clinical outcomes further supporting the favourable benefit-risk profile of ofatumumab for the treatment of RMS.
A greater treatment effect was observed in the OMB-OMB group with a numerically lower cumulative number of CDW events and numerically higher number of CDI events compared with the TER-OMB group; these results suggest an efficacy benefit that was not recovered in those initially randomised to teriflunomide and are in line with the growing body of observational studies demonstrating the benefit of early initiation of high-efficacy disease-modifying therapies on long-term disability outcomes in multiple sclerosis [20, 21].
With up to 5 years of ofatumumab treatment, > 80% of patients remained free of 6mCDW. It is now well-established that both PIRA and RAW contribute to disability accrual from early in the disease course [22, 23]. The numerically lower numbers of CDW, PIRA, and RAW events in OMB-OMB compared with TER-OMB indicate that earlier initiation of ofatumumab has beneficial effects on both relapse-independent and relapse-associated disability accrual. The proportionate benefits of ofatumumab both for disability improvement and against worsening were evident in the first 2 years of treatment, but even if the survival curves flattened with increasing time of treatment in both groups, the overall difference between OMB-OMB and TER-OMB remained visible.
The overall low rates of 6mCDW with ofatumumab treatment for up to 5 years, combined with the sustained efficacy on relapse rates and profound suppression of MRI lesion activity, resulted in 9 of 10 patients achieving NEDA-3 in both the OMB-OMB and TER-OMB groups during year 5. In the OMB-OMB group, 80% of patients achieved NEDA-3 during year 2, and this increased to 93.4% during year 5. Conversely, only 35% of patients in the TER-OMB group achieved NEDA-3 during year 2; however, the annual rates of NEDA-3 in the TER-OMB group substantially increased after switching to ofatumumab. Achieving NEDA-3 during the first 2 years of treatment has been reported to confer a lower risk of long-term disability, reinforcing the importance of early initiation, and continued use of high-efficacy therapy [24, 25].
Brain volume loss, a surrogate measure for neurodegeneration, is associated with the accrual of disability in patients with multiple sclerosis [26]. There was a lower rate of ABVC in the OMB-OMB versus TER-OMB group during the core period, followed by a similar low ABVC rate in both groups in the extension period when all patients were switched to ofatumumab (see Table S6). This observation, combined with the statistically significantly lower cumulative PBVC at year 5 in the OMB-OMB versus TER-OMB group, suggests that in the TER-OMB group during the core period, there is brain volume loss that cannot be recovered even after switching to ofatumumab (see Figure S13). Moreover, the PBVC of < 1.5% from baseline to year 5 observed in the OMB-OMB group (equivalent to a PBVC of approximately 0.3% on average per year) is in line with the rate of brain volume loss reported in monocentric studies in healthy individuals, with mean annual decreases in whole brain volume ranging between approximately 0.2 and 0.4% [27, 28].
The safety profile of ofatumumab was consistent with previous findings [10, 12] and was sustained for up to 5 years in patients with RMS, with no new safety signals identified. EAIRs of AEs and SAEs were consistent with the rates reported in the double-blind ASCLEPIOS I/II trials [10]. The most commonly reported serious infection was COVID-19. Of the nine deaths recorded, all of which occurred during the extension period, four were attributed to COVID-19/COVID-19 pneumonia during the COVID-19 pandemic, and none were reported by investigators as related to ofatumumab. The ongoing ALITHIOS study will continue to gather data on any longer-term effects of ofatumumab on IgG and IgM levels, infection rates, and infection outcomes. The rate of malignancies remained low with up to 5 years of ofatumumab treatment, also in line with previous reports [11, 12]. The safety profile of patients who had completed 5 years of treatment at data cutoff was also consistent with the known safety profile of ofatumumab with no new safety signals.
Serum IgG levels remained stable with up to 5 years of ofatumumab treatment and remained above the LLN in 98% of patients at all assessments. Although IgM levels declined, the mean IgM levels remained above the LLN and were above the LLN at all assessments in 69.4% of patients. These results are also consistent with previous reports [10, 12, 16]. Lymphocyte and neutrophil levels remained stable over time. The long-term preservation of immunocompetence suggested by these results is strengthened by the substantial rates of serological response in patients with COVID-19 immunisation events (> 60% in patients with COVID-19 booster vaccinations or breakthrough infections; see Supplementary Materials and Table S10).
Limitations of the results include a potential for attrition bias and the open-label nature of the extension study. Data presented in this study are based on a population that was selected according to the ASCLEPIOS I/II inclusion/exclusion criteria to represent a population suitable for phase 3 trials/regulatory purposes; as such, although this population reflects a broad bracket of the population of individuals with RMS, it may not completely reflect the entire population seen during routine clinical practice, including those older than 55 years and those with significant comorbidities. Finally, the inherent lack of a control group limits the ability to compare outcomes with other studies, other than those already included in the discussion.
Conclusion
The results presented here reinforce the long-term (up to 5 years) safety and efficacy of ofatumumab for treatment of RMS and its favourable benefit-risk profile [5, 10, 12]. Findings also support the early initiation of ofatumumab in patients with RMS to control overall disease activity, reduce long-term disability accrual, and slow brain volume loss.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the study participants, investigators, and staff at participating sites for supporting the conduct of the study.
Medical Writing, Editorial, and Other Assistance
The authors thank Vernice van der Merwe (Ashfield MedComms, an Inizio company), and Saimithra Thammera and Paul Coyle (Novartis) for providing medical writing support, which encompassed writing of the manuscript, formatting, referencing, preparation of tables and figures as per journal guidelines, and incorporating the authors’ revisions and finalizing the draft for submission, all under the direction of the authors. This support was funded by Novartis Pharma AG. All authors edited the manuscript for intellectual content, provided guidance during manuscript development, and approved the final version submitted for publication.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval to the version to be published. All authors are responsible for intellectual content and data accuracy.
Author Contributions
Conception and design of the work was done by Alit Bhatt, Jing Xi, Xixi Hu, Rebecca Piccolo, and Ibolya Boer. Stephen L. Hauser, Jeffrey A. Cohen, Jérôme de Sèze, Sven G. Meuth, Paul S. Giacomini, Jin Nakahara, Celia Oreja-Guevara, Derrick Robertson, Sibyl Wray, Alit Bhatt, Xixi Hu, Jing Xi, Rebecca Piccolo, Valentine Jehl, Roseanne Sullivan, Ibolya Boer, Heinz Wiendl and Ludwig Kappos contributed to data analysis and interpretation, drafting/revision of the article, and final approval of the version to be published.
Funding
The study was funded by Novartis Pharma AG, Basel, Switzerland. The study sponsor participated in the design and conduct of the study, data collection, data management, data analysis and interpretation, and preparation, review, and approval of the manuscript. Novartis Pharma AG also sponsored the publication of this manuscript along with its associated rapid service fee.
Data Availability
Trial data are available on reasonable request, provided the reason for the request is in line with current ethical and intellectual property requirements surrounding the use of data. Requests should be directed through ClinicalStudyDataRequest.com.
Declarations
Conflict of Interest
Stephen L. Hauser currently serves on the scientific advisory boards of Accure, Alector, and Annexon. He has previously consulted for BD, Moderna, NGM Bio, and Pheno Therapeutics and served on the Board of Directors of Neurona. Dr. Hauser also has received travel reimbursement and writing support from F. Hoffmann-La Roche and Novartis AG for anti-CD20 therapy–related meetings and presentations. Grants: NIH/NINDS (R35NS111644), NMSS (SI-2001-35701), and Valhalla Foundation. Jeffrey A. Cohen received personal compensation for consulting for Astoria, Bristol-Myers Squibb, Convelo, and Viatris, as well as chairing a DSMB for Celltrion. Jérôme de Seze received personal compensation from Alexion, Biogen, F. Hoffmann-La Roche Ltd, Sanofi, LFB, Merck, Novartis, Horizon-Amgen, Argenx, and UCB. Sven G. Meuth has received honoraria for consulting from Alexion, Almirall, Amicus Therapeutics Germany, Bayer Healthcare, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. He received a research grant from German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Centre for Clinical Studies (IZKF) Muenster, German Foundation Neurology and Alexion, Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. Paul S. Giacomini has received honoraria for consulting, speaking, and advisory board participation from Actelion, Alexion, Biogen Idec, Bristol Myers Squibb-Celgene, EMD Serono, Genzyme-Sanofi, Innodem Neurosciences, Novartis, Pendopharm, Roche, and Teva Neuroscience. Jin Nakahara received speaker honoraria from AbbVie, Alexion, Astellas, Biogen, Chugai, CSL-Behring, Daiichi-Sankyo, Eisai, Fujimoto Pharma, JB, Mitsubishi-Tanabe, Novartis, Otsuka, Sanofi, Sumitomo Dainippon, and Takeda. He is acting as a paid consultant for Alexion, Biogen, Chugai, Mitsubishi-Tanabe, and Novartis. His research is supported by AbbVie, Biogen, Böehringer-Ingelheim, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, JB, Keio University, Kyowa-Kirin, Mitsubishi-Tanabe, MEXT, MHLW, MSD, Otsuka, Pfizer, Shionogi, Sumitomo Dainippon, Takeda, and Tsumura. Celia Oreja-Guevara has received fees for lectures and consultations from Biogen Idec, Celgene, Sanofi-Genzyme, Novartis, BMS, Jannsen, Roche, Merck, and Teva. Derrick Robertson has received fees for consulting, contract research, and speaker bureau from Biogen, Celgene, EMD Serono, Genentech, Sanofi Genzyme, Janssen, and TG therapeutics; consulting and speaker bureau from Bristol Myers Squibb, Horizon, and Alexion; consulting and contract research from Novartis; consulting from Greenwich Biosciences; and contract research from GW Pharmaceuticals, PCORI, Atara Biotherapeutics, and CorEvitas. Sibyl Wray received fees for consulting and advisory boards for Biogen, Celgene, and EMO Serano; speaker bureaus from Biogen, Celgene, EMO Serano, Genentech-Roche, and Sanofi-Genzyme; and research support from Biogen, Celgene, EMO Serono, Genentech-Roche, Novartis, Receptos, Sanofi-Genzyme, and TG Therapeutics. Heinz Wiendl declares that he has acted as a member of the Scientific Advisory Boards of Alexion, Argenx, Biocryst, Bristol Myers Squibb, Cellerys, Galapagos, Janssen, Merck, Novartis, Sandoz-Hexal, and Uniqure. He also declares that he has received speaker honoraria and travel support from Alexion, Biogen, Bristol Myers Squibb, EPG Health, Genzyme, Merck, Neurodiem, Novartis, Ology, Roche, Teva, and WebMD Global and acts as a paid consultant for AbbVie, Actelion, Argenx, Biogen, Bristol Myers Squibb, and EMD Serono. He is acting as a paid consultant for Actelion, Argenx, BD, Bristol Myers Squibb, Dianthus, EMD Serono, EPG Health, Fondazione Cariplo, Gossamer Bio, Idorsia, Immunic, Immunovant, Inmune Bio, Syneos Health, Janssen, LTS, Merck, NexGen, Novartis, Roche, Samsung, Sangamo, Sanofi, Swiss Multiple Sclerosis Society, Toleranzia, UCB, Viatris, VirBio, and Worldwide Clinical Trials. His research is funded by Alexion, Amicus Therapeutics, Argenx, Biogen, CSL Behring, F. Hoffmann-La Roche, Genzyme, Merck, Novartis, Roche, and UCB. Ludwig Kappos’ institution (University Hospital Basel) has received the following exclusively for research support: Steering committee, advisory board and/or consultancy fees from Biogen, EMD Serono Research and Development, Genentech, Janssen, Novartis, Clene Nanomedicine Inc., Bayer, Bristol Myers Squibb, Celltrion Inc., Eli Lilly (Suisse) SA, Galapagos NV, Kiniksa Pharmaceuticals, Merck Healthcare AG, Minoryx and Santhera, Neurostatus UHB AG, Roche, Sanofi, Santhera Pharmaceuticals, Shionogi BV, Wellmera AG, and Zai Lab; speaker fees from Bristol Myers Squibb, Janssen, Novartis, Roche, and Sanofi; grants from the European Union, Innosuisse, Merck Healthcare AG, Novartis, and Roche; and testimony. Alit Bhatt, Xixi Hu, Jing Xi, Rebecca Piccolo, Valentine Jehl, Roseanne Sullivan, and Ibolya Boer are employees of Novartis.
Ethical Approval
These clinical studies were designed and implemented, executed, and reported in accordance with the International Conference on Harmonisation Tripartite guidelines for Good Clinical Practice [17] with applicable local regulations (including European Directive 2001/20/EC, US CFR 21), and with the ethical principles laid down in the Declaration of Helsinki [18]. The protocol was approved by an institutional review board or ethics committee at each trial site. All the patients or their legal representatives provided written informed consent before commencing trial-related procedures.
Footnotes
Prior Presentation: The results covered here have been partially presented as congress abstracts and posters at the American Academy of Neurology (AAN), 22–27 April 2023, Boston, USA, European Academy of Neurology (EAN), 1–4 July 2023, Budapest, Hungary; and the Joint Congress of Americas Committee for Treatment and Research in Multiple Sclerosis and the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) Meeting, 11–13 October 2023, Milan, Italy.
References
- 1.Kesimpta Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf. Accessed December 12, 2024.
- 2.Kesimpta. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf. Accessed December 12, 2024.
- 3.Novartis Kesimpta® six-year efficacy data show substantial benefits in recently diagnosed treatment-naïve people with relapsing multiple sclerosis. https://www.novartis.com/us-en/news/media-releases/novartis-kesimpta-six-year-efficacy-data-show-substantial-benefits-recently-diagnosed-treatment-naive-people-relapsing-multiple-sclerosis. Accessed December 12, 2024.
- 4.Novartis. Data on file. Kesimpta PSUR. Sep 2024.
- 5.Hauser SL, Kappos L, Bar-Or A, et al. The development of ofatumumab, a fully human anti-CD20 monoclonal antibody for practical use in relapsing multiple sclerosis treatment. Neurol Ther. 2023;12:1491–515. 10.1007/s40120-023-00518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser SL, Bar-Or A, Cohen JA, et al. B-cell depletion and efficacy outcomes with ofatumumab: subgroup analysis from the pooled phase 3 ASCLEPIOS I and II trials. Poster presented at: 72nd American Academy of Neurology Annual meeting; ePoster P7-9-013.
- 7.Bar-Or A, Grove RA, Austin DJ, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: the MIRROR study. Neurology. 2018;90:e1805–14. 10.1212/WNL.0000000000005516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selmaj K, Cree BAC, Barnett M, Thompson A, Hartung HP, et al. Multiple sclerosis: time for early treatment with high-efficacy drugs. J Neurol. 2024;271:105–15. 10.1007/s00415-023-11969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iaffaldano P, Lucisano G, Caputo F, et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord. 2021;14:17562864211019574. 10.1177/17562864211019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383:546–57. 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 11.Gartner J, Hauser SL, Bar-Or A, et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: results from ASCLEPIOS I and II. Mult Scler. 2022;28:1562–75. 10.1177/13524585221078825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser SL, Zielman R, Das Gupta A, et al. Efficacy and safety of four-year ofatumumab treatment in relapsing multiple sclerosis: the ALITHIOS open-label extension. Mult Scler. 2023;29:1452–64. 10.1177/13524585231195346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-Or A, Wiendl H, Montalban X, et al. Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study. Mult Scler. 2022;28:910–24. 10.1177/13524585211044479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kira JI, Nakahara J, Sazonov DV, et al. Effect of ofatumumab versus placebo in relapsing multiple sclerosis patients from Japan and Russia: phase 2 APOLITOS study. Mult Scler. 2022;28:1229–38. 10.1177/13524585211055934. [DOI] [PubMed] [Google Scholar]
- 15.ClinicalTrials.gov. NCT03650114. Long-term safety, tolerability and effectiveness study of ofatumumab in patients with relapsing MS (ALITHIOS). https://clinicaltrials.gov/ct2/show/NCT03650114. Accessed May 7, 2025.
- 16.Hauser SL, Cross AH, Winthrop K, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler. 2022;28:1576–90. 10.1177/13524585221079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Conference on Harmonisation. ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med. 2001;47:45–50. [PubMed] [Google Scholar]
- 18.World Medical Association. WMA Declaration of Helsinki: ethical principles for medical research involving human subjects. 2006 https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 7 Mar 2025.
- 19.U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 5. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Published November 27, 2017. Accessed December 12, 2024.
- 20.He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19:307–16. 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 21.Cobo-Calvo A, Tur C, Otero-Romero S, et al. Association of very early treatment initiation with the risk of long-term disability in patients with a first demyelinating event. Neurology. 2023;101:e1280–92. 10.1212/WNL.0000000000207664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lublin FD, Haring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147–61. 10.1093/brain/awac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cree BAC, Hollenbach JA, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85:653–66. 10.1002/ana.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015;72:152–8. 10.1001/jamaneurol.2014.3537. [DOI] [PubMed] [Google Scholar]
- 25.Alonso R, Casas M, Lazaro L, et al. Achieving no evidence of disease activity-3 in highly active multiple sclerosis patients treated with cladribine and monoclonal antibodies. Mult Scler J Exp Transl Clin. 2023;9:20552173231154710. 10.1177/20552173231154712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cagol A, Schaedelin S, Barakovic M, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. 2022;79:682–92. 10.1001/jamaneurol.2022.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:93–9. 10.1136/jnnp-2014-309903. (Epub 2015 Apr 22 PMID: 25904813). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uher T, Vaneckova M, Krasensky J, et al. Pathological cut-offs of global and regional brain volume loss in multiple sclerosis. Mult Scler. 2019;25:541–53. 10.1177/1352458517742739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Trial data are available on reasonable request, provided the reason for the request is in line with current ethical and intellectual property requirements surrounding the use of data. Requests should be directed through ClinicalStudyDataRequest.com.