Abstract
Introduction
Myasthenia gravis, a rare autoimmune disorder characterized by muscle weakness and fatigue, it is a mainly B-cell mediated condition with antibodies directed against the acetylcholine receptor or functionally related molecules at the neuromuscular junction. Corticosteroids are still the most used treatment, as they are cheap and characterized by a rapid response. However, their long-term administration is associated with frequent and often severe side effects.
Methods
We used the Expert Opinion methodology: a panel of eight neurologists, known to be experts in the management of MG patients, and one specialist in pharmacoeconomics, were brought together to discuss clinical relevant issues about the use of corticosteroids in MG.
Results
Increasing doses of corticosteroids may temporarily exacerbate the symptoms of MG and clinical exacerbations can lead to severe consequences. In addition, prolonged chronic corticosteroid therapy carries a burden in terms of indirect costs due to side effects, which has prompted strategies to obtain the maximum benefits with minimal side effects.
Conclusion
The panel concludes that, in the near future, therapeutic strategies based on the use drugs with better tolerability and potentially lower direct and indirect costs, will be necessary.
Keywords: Corticosteroids, Myasthenia gravis, Management
Key Summary Points
| Why carry out this study? |
| To date, there are no clear guidelines for steroid therapy in myasthenia gravis in terms of starting, tapering, and chronic management. |
| An Expert Opinion methodology has been used to discuss clinical relevant issues about the use of corticosteroids in myasthenia gravis. |
| What was learned from this study? |
| Starting therapy with corticosteroids may temporarily exacerbate the symptoms of myasthenia gravis, leading to severe consequences and chronic steroid therapy carries a burden in terms of indirect costs due to side effects in myasthenia gravis. |
| Non-steroidal immunosuppressant agents have allow to reduce steroid intake with relevant side effects. |
| In the era of the new therapies for myasthenia gravis, these recommendations can help patients to achieve the best therapeutic goals. |
Introduction
Myasthenia gravis (MG) is an autoimmune disease caused by auto-antibodies to acetylcholine receptors or functionally related molecules at the postsynaptic membrane of the neuromuscular junction [1, 2]. MG is a rare disorder with an annual incidence of around 1.7–21.3 per 1 million person-years and a prevalence of 15–179 per 1 million people [3]. In Italy, real-world data have suggested that the prevalence of MG ranges between 13 and 293 per 1 million people [4].
The clinical features of MG are muscle weakness and fatigability and, usually, patients’ strength is higher in the morning and worsens as the day progresses [1, 2]. Skeletal, extraocular, and bulbar muscles are variably involved in MG. In about 20% of patients with MG, symptoms are confined to ocular manifestations [5], while a generalized onset is seen in roughly half of patients [6].
The clinical manifestations of MG typically exhibit temporal variability and, despite the considerable understanding and availability of diverse therapeutic modalities, the identification of an optimal treatment in individual patients may remain complex. The universal adoption of best-practice standards presents a significant challenge, particularly given the potential advance of therapeutic interventions that can substantially improve MG patient outcomes [1, 2].
Patients with MG can be classified into subgroups depending on the extent of clinical symptoms, age at onset, types of auto-antibodies, and thymus abnormality [1, 2].
These subgroups have differences in epidemiology, pathophysiology, severity of symptoms, and response to therapy [5]. Around 85% of patients with MG have antibodies against the acetylcholine receptor (AChR+), while a small fraction display antibodies against muscle-specific kinase, MuSK+, (about 10%) or lipoprotein-receptor-related protein 4 (LRP4) (1–3%) [7]. Patients negative to any of the previous antibodies (2–13%) are referred to as triple seronegative MG [8].
Advancements in MG treatment have led to an overall improvement of clinical benefit. Nevertheless, a notable proportion of patients fails to achieve sustained disease control, thus a persistent challenge in optimizing therapies tailored to specific patient subgroups exists [9, 10].
Ideally, the aim of therapy for MG would be the complete remission of symptoms or at least the presence of minimal symptom expression, which can be achieved while receiving treatments and maintained after treatment weaning. A step-by-step approach for the treatment of generalized MG has been advocated, based on improving neuromuscular transmission by acetylcholinesterase inhibitor (pyridostigmine) and the modulation of the immune reactions at the basis of the disease pathogenesis. Thymectomy in selected cases, corticosteroids (CSs), and non-steroidal immunosuppressors, used as steroid sparing agents, are the common treatment strategies [1, 2].
Nowadays, CSs continue to be the mainstay of immunotherapy of MG being prescribed in 54.5% of patients in the first year and decreasing to 44.6% at the third year; as observed in a recent real-world Italian study [11]. CSs are associated with a relatively rapid clinical response within 2–4 weeks [12, 13], and are widely available at low cost. However, long-term administration of CSs is associated with side effects leading to substantial clinical and economic impact [14, 15]. Common side effects include insomnia, mood alterations, and impaired glucose tolerance, as well as increased risks of severe adverse effects such as osteoporosis with high risk of pathological fractures, glaucoma, cataracts, infections, cardiovascular events, and iatrogenic Cushing syndrome [10, 15, 16]. In some cases, iatrogenic side effects may impose a significant burden on patients’ quality of life and lifestyle [17–19]. While a combination of immunosuppressive and supportive, (e.g., physical and psychological support) therapies are able to control disease manifestations and quality of life, many patients still experience MG symptoms [1, 16, 20].
Consensus on management of MG includes only limited guidance on the use of CSs [7, 21, 22]. Given that CSs are still in widespread use and remain a pillar of MG treatment, a group of experts met to formulate specific, unanimously agreed recommendations for their use. The overall aim is to expand existing recommendations to help clinicians in daily practice and to shed light on possible uncertainties regarding the prescription and use of CS therapies. The specific recommendations cover initial administration of CSs, their use as maintenance therapy, when to stop them, considerations in specific subgroups and prevention of complications.
Aim of the Study
To improve knowledge about the use of corticosteroids in clinical practice and make recommendations regarding their prescription, with the aim of improving patients health-related quality of life (QoL).
Materials and Methods
This consensus statement does not involve human participants, patient data, or interventional research requiring formal ethical approval. The recommendations presented are based on expert opinion and a review of the existing literature.
The study was organized with the following process, shown in Fig. 1, which was run over a period of 6 months in 2022. The expert panel consisted of 8 neurologists and 1 specialist in pharmacoeconomics.
Fig. 1.
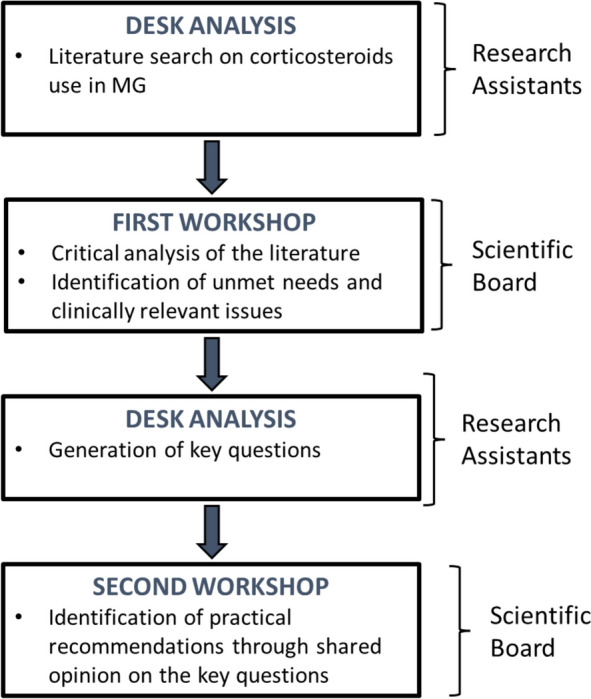
Overall flow of the process employed in the study
Prior to the first meeting, literature searches were carried out on PubMed to identify relevant publications on the use of CSs in MG considering: (1) efficacy, (2) safety, (3) use in specific subgroups (elderly, pediatric patients, patients with comorbidities, pregnancy and breastfeeding), (4) costs of therapy, (5) quality of life,(6) monitoring during chronic therapy, (7) infectious risk, and (8) use with vaccines.
Details on the search string used for each selection process are detailed in the Figs. 1 and 2.
Fig. 2.
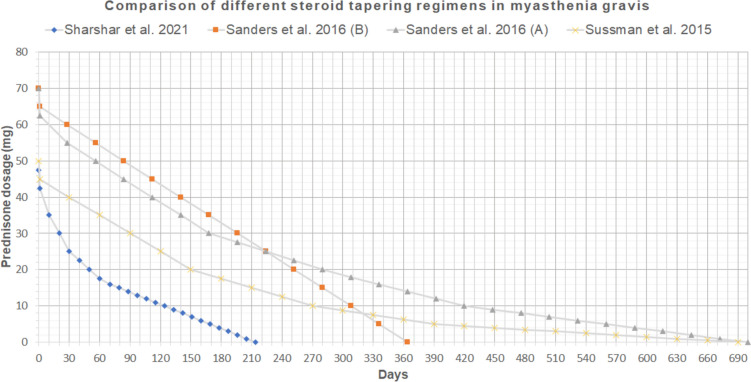
Comparison of different steroid tapering regimens in MG [20, 49, 50]
In the first workshop, the discussion was centered on a critical analysis of the international and national literature on the use of CSs for MG in relation to the expert group’s clinical practice in real life, which enabled the identification of unmet needs and clinically relevant issues for disease management.
Based on this analysis, a set of key questions was developed.
In the second workshop, the expert panel developed practical recommendations through shared opinion on the key questions formulated. During these web-based meetings, workshop discussion sessions were undertaken by the expert panel and the outputs of the discussion are reported in Tables 1, 2, 3 and 4, and in the Results section.
Table 1.
Recommendations on initial administration of corticosteroids
| Choice of corticosteroid (CS) |
• The therapeutic decision of starting corticosteroids must be tailored to the individual patient [23–25]; if the patient responds to anticholinesterase alone, a CS is not needed [7] • Among the available CS therapies, prednisone represents the good choice in terms of ease of use, modularity, and anti-inflammatory activity [25]; for these reasons prednisone is considered as the first-choice in daily clinical practice [7, 23, 25, 26]; in selected cases, the use of a different CS agent can be considered, as long as it is administered in equivalent doses[23] • In real-life practice in Italy/Europe, intravenous methylprednisolone is rarely used • The use of CSs in the treatment of ocular MG must be guided by the clinician [25, 27, 28], regardless of the presence of detectable antibody • Consider administering immunoglobulins or performing plasmapheresis before starting CS therapy in patients who are critically-ill (e.g., severe bulbar MG) to avoid the clinical worsening observable in clinical practice when starting CS [7, 22, 29] |
| Initial dose of corticosteroids |
• The starting dose of prednisone should be 1–1.5 mg/kg/day until substantial clinical improvement is achieved (a dose which requires hospitalization), then decreasing to the recommended dose of 0.75–1 mg/kg/day [7] • For treatment of ocular MG, a lower dose should be used (0.5 mg/kg/day)[23] |
| Route of administration |
• The use of an intravenous CS bolus (500/1000 mg of methylprednisolone) is not adequate for a chronic treatment of MG in myasthenic patients • In case of severe dysphagia, administer the CS through a nasogastric tube [30] • it is the opinion of the panel of experts that in patients who refuse a nasogastric tube dexamethasone may represent a valid option |
| Posology |
• Administer a single dose of prednisone in the morning, and doses should not be divided throughout the day • To manage short-term adverse events related to CS therapy, an alternate day dosing schedule may be used in selected patients to reduce swelling [31, 32] |
Table 2.
Recommendations for use as maintenance therapy in clinically controlled patients
| Modification of treatment with corticosteroids (CSs) when clinical remission is achieved |
• A key criterion to be followed when deciding how to taper and maintain clinical remission is that the risk for and presence of side effects should be carefully weighed against the risk of relapse[33, 34] • In patients with ocular MG, faster reduction of therapy can be considered, though personal reactions should be always evaluated • In patients with a history of relapse and bulbar symptoms, dose reduction should be slow because the risk of severe relapse may outweigh the risk of developing adverse effects [34] • Upon achieving clinical remission, the abrupt discontinuation of therapy is contraindicated. Reduction of the dose of CSs must be progressive and slow, but modulated in consideration of various factors such as the starting dose when reduction is begun, and the patient's clinical history (e.g., in the elderly patients, in those with comorbidities, and in those with established combination with NSIST are situations in which faster dose reduction can be sought) [7, 34–36] |
| Dose and timing of tapering |
• Although there may be variability in the rate of dose reduction based on various factors and/or the patient's clinical history, the reduction should not exceed a 10% dose decrease every 2 weeks. Tapering is subject to clinical evaluation: there is no predetermined timing for tapering, and the clinician must evaluate how the patient responds • Maximum treatment time: with CS therapy the maximum benefit is usually achieved after 40–60 days [26, 37]. After this period of time, it is necessary to evaluate the patient and understand if a stable clinical condition has been reached. If the patient has reached the maximum benefit, tapering can begin. Otherwise, it is possible to intervene with “immunomodulating” therapies, i.e. a course of immunoglobulins or plasmapheresis • Criteria to be followed to identify the optimal time for CS reduction: o start tapering when patients are on a stabilized clinical condition o Initiate the reduction considering adverse events and comorbidities in the individual patient o In AChR + MG patients start reduction of the CS on the basis of the patient's need for anticholinesterase (the same is usually not applying to MuSK + patiernts); o it is possible to start with a 10% reduction on daily CS dose, and then change to administration on alternate days (with careful monitoring for possible overtreatment) [36] • The speed of tapering must be adequate to the patient’s condition and tolerability, while monitoring the maintenance of the benefit achieved • The reduction should be made considering the daily treatment schedule and then to administration on alternate days [31] |
| Minimum effective dose as maintenance therapy |
• The minimal effective dose is empirically established • An acceptable prednisone maintenance dose is approximately 5 mg to 25 mg every other day (2.5 to 12.5 mg per day) [26, 37–39] • In patients on CS monotherapy, the minimum acceptable effective dose depends on individual patient. In case of recurrence of symptoms, increase the dose again and the optimal dose depends on the patient response • If patients are not completely controlled by a CS monotherapy, consider to add a NSIST [7] |
| Optimization of long-term therapy: when to use a CS-sparing agent |
• CS-sparing strategies must be personalized to the individual patient; for example: o Patients who achieve a satisfactory clinical outcome and present no risk factors can continue monotherapy while reducing dosages as illustrated above [24, 39] o In patients with comorbidities in whom CSs are not indicated or in case of lack of response to CSs, a sparing agent should be used as soon as possible, depending on the specific comorbidities present [7, 24, 39] |
Table 3.
Recommendations for use in specific subgroups
| Elderly patients |
• For treatment of elderly patients, use slightly lower CS dosages and carefully determine compatibility with other existing therapies [39] |
| Pediatric patients |
• Pediatric patients with onset of disease before puberty should be followed at specialized centers • Pediatric patients should initiate Vitamin D and be monitored for growth, weight, and bone density [40] • Pediatric and, primarily, adolescent patients may need psychological support to manage the stress caused by adverse events associated with steroid use [40] |
| Comorbidities |
• In patients with relevant comorbidities, a specialist should be involved in treatment (e.g., diabetologist, cardiologist, pulmonologist, ophthalmologist) [24, 39, 40] • In cancer patients with active disease and ongoing treatments, collaboration with the oncologist is recommended to agree on treatment protocols |
| Pregnancy |
• While there is limited literature, in clinical practice CS are widely used in pregnancy (not only for the treatment of MG) and there are no formal contraindications to their use [7, 20, 41] • The dose must be the minimum necessary and adapted to the clinical status of the patient; the CS should be reduced to the minimum possible tolerated dose [39] • Management of CS therapy must be agreed with the gynecologist [20, 22, 39, 42], since their negative effects may be different depending on the gestational period (in addition to the anesthetist who will follow the birth for any supplementation needed during parturition) |
| Breastfeeding women |
• The administration of a CS at low doses during breastfeeding is not contraindicated [7, 20], but its use should be discussed with a neurologist, gynecologist, and pediatrician |
Table 4.
Recommendations for prevention of complications
| Corticosteroids (CSs) and vaccinations |
• Discontinuation of CSs prior to vaccination does not appear to be necessary [22] • live attenuated virus vaccines are contraindicated in the course of CS therapy • CS administration is compatible with inactivated virus or mRNA vaccines (e.g., Covid-19 mRNA vaccine)[22] • The administration of seasonal flu vaccine in myasthenic patients does not induce additional problems compared to the general population [22, 40] • There are no studies on travel vaccines (e.g., yellow fever), but the diseases for which patients are vaccinated are much more dangerous than myasthenia |
| Risk of infection with CSs |
• The risk of infections, and therefore the rationale for requesting infectious disease consultation before starting CS therapy in a patient with MG is to be assessed on a case-by-case basis [22, 39, 40] • At the start of treatment with a CS (especially at high doses), infectious disease screening is recommended to check for the presence of latent infections (e.g., quantiferon test for TB, markers for HCV, HBV, VZV) • Hospitalized patients, especially if in an intensive care unit, the elderly patients, and those with other diseases, are at greater risk of infections [38] in relation to chronic usage of CS, particularly when associated with NSIST • The patient’s personal history of infection should be considered before starting CS [39]; a particular attention should be given to possible non-clinical evident tuberculosis making reasonable to evaluate patients with chest X-rays in addition to specific laboratory tests |
| Monitoring during chronic CS therapy |
• Monitoring should include routine blood chemistry tests (blood count, glycemia with glycosylated hemoglobin, organ function, ion levels), blood pressure, body weight, and abdominal circumference [27] • Specialist visits are recommended in patients on prolonged therapy with CS: ocular, endocrinological, cardiological (especially in elderly subjects), and dermatological examinations [7, 20, 39, 40] • Frailty MG patients, such as elderly patients, women close to menopause, etc.) should be referred to MG specialized centers where a multidisciplinary teams can address and approach the use of chronic CS treatment more properly on the basis of their experience [24, 27, 43, 44] |
The key questions formulated by the working group were divided into four areas: (1) initial administration of CSs; (2) use as maintenance therapy in clinically controlled patients; (3) considerations in specific subgroups (elderly, pediatric patients, patients with comorbidities, pregnancy and breastfeeding); and (4) prevention of complications. For each area the current evidence is summarized followed by specific recommendations of the working group (Tables 1, 2, 3, and 4).
Results and Discussion
Initial Administration of CSs
The main debated points concerning the initiation of steroid therapy are: (1) molecule choice, (2) mode of administration of the drug and (iii) starting dosage.
Molecule Choice
The molecule of choice, according to the experts, given its pharmacological characteristics and easiness of use, is prednisone.
Mode of Administration
According to the panel of Italian experts, in the majority of cases treatment with oral prednisone is preferred over intravenous methyl prednisolone (IVMP), as oral CSs have been demonstrated to induce a clinical improvement, usually during the first 4–8 weeks [35]. However, IVMP can be used in hospitalized patients with swallowing difficulty and under strict surveillance.
Starting Dosage
The initial steroid dosage is particularly critical, as starting with high dosages can induce a transient initial aggravation of myasthenic symptoms, may influence the treatment speed of action and the appearance of early side effects.
According to a systematic review by Lotan et al. [45], whose results however are derived from low quality heterogeneous retrospective studies (the only ones available in the literature), the risk of exacerbation of myasthenic symptoms after the start of treatment varies with the type and dose of CSs; high doses are more frequently associated with exacerbations which, although mild to moderate in most cases, require close clinical monitoring (even in a hospital setting) in these patients.
S high dose approach should be carefully considered for patients with bulbar involvement, older age, generalized MG and thymoma, as they are at higher risk for complications, including respiratory failure [23, 45].
IVMP can be useful in particular cases because it offers the opportunity of parenteral administration. The equivalent ratio methylprednisolone/prednisone is 0.8.
Randomized studies comparing low-dose versus high-dose CS therapy initiation are lacking, but there are studies encouraging the initiation of low dose therapy. In particular, in a 2-year observational study, 63 patients with generalized MG (MGFA II–IV) receiving low-dose initiation and slowly incrementing CS therapy after thymectomy had a favorable response, with an early response to low-dose prednisolone therapy predicting a better long-term outcome [46]. However, some concerns were raised by the panel. In particular, panelists shared the opinion that starting with a low-dose CSs could imply that some patients may receive a sub-optimal dosage based on the disease severity. Moreover, it may be difficult to discriminate whether the patient is CS refractory or the dosage ineffective. Finally, the adoption of an incremental dose schedule might expose the patient to a hypothetical risk of clinical deterioration at each increment. On this basis, the panel agreed that CSs should be initiated at a full dose of 0.75–1 mg/kg/day in a patient naïve to immunosuppression [7]; this is of particular relevance in patients with a severe axial/bulbar involvement who are usually hospitalized (Table 1). When the patient is managed as an outpatient (which is actually the most common case in patients with mild/moderate disease), it is more appropriate to start steroids at lower dosages with an increasing schedule up to the therapeutic dose [1, 7]. The association of rescue therapies such as IVIg or plasma exchange, as prevention or treatment for possible initial steroid-related deterioration, could be considered [7, 45, 47] (Table 1). In patients with ocular MG, a lower dose of CS can be used, usually 25 mg/day or 0.5 mg/kg/day [23].
It is important to note that the decision to start with an optimized full or incremental dose is not evidence-based and therefore the choice must be made considering the various variables such as the patient setting (inpatient or outpatient), patients’ comorbidities and the disease severity, with particular reference to respiratory function and bulbar deficits.
Use as Maintenance Therapy in Clinically Controlled Patients
Discussion on chronic CS treatment was focused on the following points: (1) chronic treatment schedule; (2) association with non-steroidal immunosuppressive agents (NSIST); (3) tapering schedule of steroid therapy in clinically stabilized patients; and (4) minimum effective dose. All the recommendations developed by the panel on this issue are reported in Table 2.
On a chronic schedule, due to the prolonged half-life, prednisone may be used on an alternate-day treatment protocol; once a stable clinical condition has been reached, CS can be gradually tapered to the minimum effective dose; a CS-sparing approach can be envisaged using dose tapering/de-escalation protocols [7, 24].
Considering the high rates of CS-related side effects and comorbidities, starting CS- sparing immune suppressive treatment when possible is suggested [46, 48]. There are three published studies on steroid tapering regimens in MG, and a comparison of these shown in Fig. 2, demonstrating that, depending on the starting point and the tapering strategy, the time needed to reach the treatment target, ranges from 7 months up to 2 years [20, 49, 50].
The graph describes the ideal tapering regimen recommended for an average patient weighing 70 kg. For practical reasons, regimens presented consider a total daily dose of prednisone, even though some guidelines recommend alternate day administration. Two different taper regimens were suggested in Sanders et al. [20]: taper of 10% of the dose every month (A), taper of 5 mg of prednisone every month (B).
Sharshar and colleagues performed a randomized clinical study comparing fast and slow tapering in a population of patients who received the association of CS and azathioprine showing that “rapid tapering of prednisone appears to be feasible, well tolerated, and associated with a good outcome”. In the best-case scenario, prednisone was discontinued before day 200 in a 60-kg patient [49].
Neurologists usually taper CSs slowly once symptom control has been achieved, and the dose is tapered every month to the minimum effective dose [47]. According to the panel, for patients on CS monotherapy, the minimum acceptable effective dose depends on individual characteristics and choosing an alternative immunosuppressant as an add-on if a high minimum effective dose is required [7].
Despite the absence of specific protocols or standardized guidelines for the downscaling of CSs, there is broad consensus among experts on the need for slow tapering. As a result, an individualized approach, tailored to each patient’s clinical needs and circumstances, has been recommended. However, this approach lacks uniformity across centers, highlighting variability in clinical practices.
The panel also agrees that the reduction of the CS dosage is generally more effective when performed progressively but slowly, and adjusting the dose accordingly. In addition, the reduction should be performed by taking into consideration adverse events, comorbidities, and disease duration in the individual patient. Particular concern should be given to patients with a history of relapse and bulbar symptoms in the elderly and in those at high risk of osteoporosis. On the other hand, with regard to patients on another immunosuppressant, it is possible to reduce CSs more quickly, as demonstrated by Sharashar et al. [49].
Long-distance scheduling of treatment tapering without periodic evaluation of patients should be discouraged, as well as personal initiatives by patients on treatments. In this respect, it is essential that the patient is informed on the timing of treatments from the beginning of therapy.
It should also be noted that the recent development of biologics with more targeted mechanisms of action and improved safety profiles [51–54] aims to address a critical clinical gap, enabling the reduction of CS therapy to doses below the Cushing threshold. This represents a pivotal step toward minimizing the adverse effects associated with prolonged CS use, while maintaining effective disease control in MG patients.
Use in Specific Subgroups of MG Patients
All recommendations developed by the expert panel on CS use in specific subgroups are reported in Table 3.
According to the panel opinion, it is important to consider patients with neoplastic disease as a special population, including not only patients with high grade thymoma but also those with other tumors. Some chemotherapy agents have an immunosuppressive effect and can help in the treatment of myasthenia; however, other immunological treatments for tumor, such as some biologics and immune checkpoint inhibitors, may aggravate MG, taking into consideration that the latter therapeutics are able to induce MG in non-myasthenic patients. Although the use of immunosuppressant treatments in patients with cancer is controversial and sometimes contraindicated (e.g., NSIST in patients with chemotherapy-induced bone marrow aplasia) in general, the use of CSs in these patients is allowed.
There are limited studies in the literature on the use of CSs during pregnancy. Drug treatment, as well as autoimmune comorbidities, may have an impact on both mother and fetus during pregnancy, but prednisone and prednisolone are considered to be safe to use during pregnancy [41]. In a prospective study of women with MG and newborns followed for 9 years, neither duration of illness nor therapy during pregnancy correlated with neonatal MG [39]. In another prospective study of 25 pregnant women with MG, 43.3% received CSs during pregnancy. There were no cases of fetal growth restriction or demise, although two infants did develop transient neonatal myasthenia. It has been reported that a proportion of women may experience clinical worsening of MG during pregnancy or after delivery [55].
However, a systematic review on MG and pregnancy concluded that the majority of women with MG usually have a normal course of pregnancy with favorable outcomes for both the mother and infant. It is highly recommended to plan carefully delivery setting which should be performed in a multidisciplinary context [42]. In general, in diseases other than MG, CS use during pregnancy does not appear to increase the risk of preterm birth or low birth weight, although the evidence is limited [42]. Of note, a report showed that in women with rheumatoid arthritis high-dose CS in both early and late pregnancy was associated with a higher risk of pre-term births [56].
Prevention of Complications
Long-term administration of CSs has been associated with a number of side effects including hypertension, obesity, gastric ulcer, cataracts, Cushingoid appearance, psychological disturbances, opportunistic infections, sepsis, and serum electrolyte alterations [24, 25, 49]. Moreover, long-term CS use can also result in metabolic consequences such as insulin resistance and diabetes [57]. It is recommended to monitor glycemic profile of patients, including glycated hemoglobin and insulinemia, to achieve the optimal daily steroid dosage and minimize the adverse events. The most debated points in this issue are: (1) prevention of complications; (2) risk of infection during steroid treatment; and (3) vaccination during steroid treatment.
All the recommendations developed by the panel of experts on prevention of CS complications are reported in Table 4.
All the panelists agreed that iatrogenic complications, particularly in patients with comorbidities, should be treated by single specialists, such as diabetologist, rheumatologist, cardiologist, pulmonologist, ophthalmologist, etc. [24, 39, 40].
CS treatment needs to be personalized in older patients depending on the specific comorbidities present in the individual patient; likewise the possible interaction/conflict with the drugs used for comorbidities should be taken into consideration to minimize the impact [39]. Careful consideration should be given to the impact of CS treatment on the development of diabetes, hypertension, obesity with cardiac stress and cardiac events, and osteoporosis with propensity to spontaneous fractures in elderly patients [39]. Osteoporosis should be regarded as one of the most serious AEs as it may occur even with low-dose treatment (steroid treatment ≥ 2.5 mg/day for more than 3 months, regardless of the type of steroid), and in the presence of osteoporotic risk factors, such as smoke, alcohol, prior fractures, thyroid/parathyroid disease, and age ≥ 40 years. These patients should undergo bone densitometry measurement, supplementation with calcium or Vitamin D, and should be referred to s rheumatologist/endocrinologist for appropriate pharmacological treatment since the beginning of steroid treatment [33]. Regarding the timing of bone densitometry, there is no specific guidance for patients with MG. In 1998, a consensus group from the UK recommended that, in patients receiving long-term CSs (≥ 7.5 mg daily for ≥ 6 months), dual energy X-ray absorptiometry (DEXA) scanning should be performed yearly, while bisphosphonate therapy should be administered to those receiving prednisolone ≥ 15 mg daily for ≥ 6 months independently of the DEXA results [58].
Numerous adverse effects have also been associated with CS use in pediatric patients: they include mood and behavioral disturbance, sleep disruption, acneiform eruptions, weight gain, growth restriction, hypertension, diabetes, osteoporosis, infections, and gastroesophageal reflux disease. Such adverse effects can cause psychological stress, particularly in adolescents, who should be offered psychological support. Children with myasthenia should also be initiated on vitamin D according to local guidelines and should have bone density assessment [34, 40].
Literature data on the use of CSs in myasthenia gravis and the risk of infections or use of vaccines are scarce, with evidence limited to case reports and to a few studies which do not suggest stopping CSs before vaccination. This was also reflected in the real-life clinical practice of the experts panel. While there has been controversy in the past on the efficacy and safety of influenza vaccination in MG patients treated with immunosuppressive therapy including steroids, recent studies appear to suggest that influenza vaccination is effective and that the benefits outweigh any potential risks [59–61].
In the era of COVID-19, an observational study in 99 patients with MG reported that mRNA vaccination against SARS-CoV-2 appears to be safe, with no negative impact on the disease course. However, combined therapy with prednisone and other immunosuppressive drugs correlated with a lower seroconversion ratio and a lower T-cell response [62, 63].
It is noted that vaccine recommendations for patients with neuromuscular disease under immunosuppression including CSs should receive inactivated vaccines as well as the influenza and pneumococcal vaccines, and should not be administered live attenuated vaccines [64]. Thus, MG patients on immunosuppression can be vaccinated, although those on mycophenolate mofetil, as well as those on anti-B cell monoclonal antibodies (rituximab or anti-CD20 agents), are likely not to have a proper seroconversion.
Clinicians should be aware of the tuberculosis predisposition or the possibility of reactivating a latent infections in patients with MG, as demonstrated in a cohort study of over 2300 individuals [65]. In addition, various case reports indicated that MG patients being treated with a CS and azathioprine might represent a susceptible population to Nocardia [66], or Legionella infection [67]. Since a significant association between risk of infection and chronic CS therapy is likely, it is of paramount importance to carefully evaluate risks prior to immunomodulatory therapy initiation, to consider use of screening, and to set up prophylaxis against common or serious opportunistic infections when diagnosed (i.e., tuberculosis prophylaxis, etc.) [68]. Overall, it is advisable to request an infectious disease consultation before starting CS therapy in MG patients by using a case-by-case approach [22, 39, 40]. Also, the psychological effects of CS, such as insomnia, anxiety, and depression should be taken into consideration, as they may lead to therapy discontinuation or to a substantial reduction of the doses.
Additional Considerations on the Use of CSs in MG
Direct and Indirect Costs
Considerations on costs are summarized in Table 5. No relevant results regarding the direct and indirect costs associated with the use of CSs in MG emerged from a literature search. Taking into account all the above considerations, it seems rational to prioritize treatments and procedures which will help to reduce direct and indirect costs associated with the chronic CS therapy in patients with MG, in the perspective to reduce burden of disease and to improve the activities of daily living of patients.
Table 5.
Considerations on costs and QoL
| Costs |
• The low cost of CSs does not outweigh all the problems related to their use, and adverse events related to CS use also have high economic impact [16] • Optimized CS, in consideration of possible adverse events related to its effects [16], will reduce direct and indirect costs (adequate vs. inadequate use) • The pathologies that develop following use of CS are difficult to put under control (e.g., weight gain, metabolic syndrome) and are associated with a high frequency of other chronic pathologies (e.g., diabetes, osteoporosis) [41, 57] • Iatrogenic diseases and side effects lead to increasing both direct and indirect costs, which are currently not well quantified in the literature [16]; it would be necessary to quantify these costs to better understand the exact impact of these drugs |
| Quality of life |
• CS therapy also has a negative impact on the QoL of patients which stresses the need for correct and optimized use [17–19, 30] • QoL is mainly linked to the CS dosage, as higher doses are associated with worse QoL [17–19, 30] |
Quality of Life
High doses of CSs have been significantly correlated with poor health-related quality of life (HR-QoL) compared to lower doses, as evaluated by the MG activities of both daily living and MG-QoL 15 scales [17, 19]. Moreover, factors promoting social disadvantages include severity of illness, dose and duration of prednisone, long-term treatment, depressive state, and change in appearance after treatment with oral CSs [18]. A Cushingoid appearance may have a negative effect on HR-QoL, even if other side effects of CSs are not apparent, and prednisolone ≤ 5 mg/day was found to positively impact HR-QoL and has been recommended as a treatment target [44, 69]. Glucocorticoid-induced osteoporosis can also deteriorate the QoL in patients with MG. Indeed, patients affected by osteoporosis are recommended to limit their mobility in order to prevent bone fractures. In the meantime, the consequences of osteoporosis, such as spinal or thighbone fractures, frequently alter ambulation or general motility of patients [58, 70]. Thus, reducing or even discontinuing prednisone therapy without destabilizing the disease is a major therapeutic goal in generalized MG, and can be achieved by tapering, as described earlier [49]. Considerations on QoL are summarized in Table 5.
Limitations of the Study
The main limit of this study is that it is based on the evidence-based experience of an expert group coming from a single country.
Conclusions
In the landscape of myasthenia gravis (MG) treatment, CSs have been and continue to be a cornerstone, offering significant symptom relief for a wide range of patients [1, 2, 11, 14, 21, 23]. Despite their low cost, wide availability, relatively fast action, and the experience gained over decades of use [16], it must be taken into account that MG symptoms may be exacerbated by initial CS administration or, sometimes, when increasing doses during patients follow-up, may temporarily exacerbate MG symptoms. Clinical exacerbations can be so severe as to require admission to the emergency care or hospitalization [71].
It is likely that prolonged chronic CS therapy carries a burden in terms of indirect costs due to its multiple side effects, which have prompted new strategies to obtain the maximum benefits with minimal side effects [1, 11, 14, 21, 23], particularly aimed at an optimized patient’s tailored treatment. The recent development of biologic treatments with more targeted mechanisms of action and favorable side effect profiles [47, 53, 54], already poses the question to the medical community on how to drive the change of the treatment paradigm of MG. Costs related to these new therapies are already high, requiring an accurate selection of candidate patients.
It is the opinion of the panel that the recommendations given in this article (Tables 1, 2, 3, 4) can help MG patients achieve the best therapeutic goals in the treatment of their disease, while reducing CS-related side effects, lowering the direct and indirect costs of the disease (i.e. frequent hospitalizations, recurrent rescue therapies, treatment of CS side effects, etc.), and improving patient QoL. We have to consider that, in the near future, therapeutic strategies based on the use of more effective and drugs with better tolerability and potentially lower direct and indirect costs will become part of the therapeutics of MG, and that CS will probably be adapted to the new scenario.
Acknowledgements
The content of the manuscript and decision to submit was the authors. EP and RL of the several authors of this publication are members of the European Reference Network for Neuromuscular Diseases – Project ID N° 870177.
Medical writing/Editorial Assistance
The authors of this article are grateful to Hippocrates Sintech S.r.l. for providing methodological and organizational assistance with the analysis and Helaglobe S.r.l. (Lucia Politi, Ph.D.) for medical writing and editorial support with the manuscript.
Author Contributions
Renato Mantegazza, Giovanni Antonini, Matteo Gastaldi, Rocco Liguori, Michelangelo Maestri, Elena Pegoraro, Barbara Polistena, Carmelo Rodolico and Francesco Habetswallner ideated and planned the present project. All authors participated to the meetings and approved the present manuscript.
Funding
The manuscript was funded by UCB who had no role in content development. Methodological and organizational assistant, editorial support and the journal’s rapid service fee was funded by UCB.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflicts of Interest
Rocco Liguori has received consulting fees, honoraria for lectures or presentation from Maya Idee Sud srl, ISS, Qb Group Srl, Symposium Srl, Accademia Nazionale di Medicina, AIM Italy srl, Aristea Srl and participation to advisory board fees from Argenx; Francesco Habetswallner has received honoraria for lectures or presentation, support for attending meetings or participation to data safety monitoring board/advisory board fees from Alexion, Argenx, UCB,Roche, Merck, Janssen, Lusofarmaco, Angelini, Abbvie, IPSEN, Alfa Sigma, CLS Boering, Takeda; Michelangelo Maestri has received honoraria for lectures or presentation, support for attending meetings or participation to data safety monitoring board/advisory board fees from Alexion, Argenx, UCB, Idorsia, Bruno farmaceutica, Italfarmaco; Matteo Gastaldi has received honoraria for lectures or presentation, participation to data safety monitoring board/advisory board fees UCB, Roche, Alexion, Johnson and Johnson, Argenx; Elena Pegoraro has received consulting fees, honoraria for lectures or presentation from Alexion, UCB, Biogen, Roche, Santhera, Sanofi; Barbara Polistena has received honoraria for lectures or presentation Amgen, Amicus, UCB; Carmelo Rodolico has received support for attending meetings or participation to data safety monitoring board/advisory board fees from Alexion, Argenx, Johnson & Johnson, UCB Pharma; Giovanni Antonini has received honoraria for lectures or presentation, support for attending meetings or participation to data safety monitoring board/advisory board fees from Kedrion, Takeda, Alexion, UCB, Alnylam, Argenx. Renato Mantegazza has received support for attending meetings or participation to data safety monitoring board/advisory board fees from Alexion, Argenx, Johnson & Johnson, UCB Pharma.
Ethical Statement
This consensus statement does not involve human participants, patient data, or interventional research requiring formal ethical approval. The recommendations presented are based on expert opinion and a review of the existing literature.
References
- 1.Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375:2570–81. 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 2.Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14:1023–36. 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 3.Carr AS, Cardwell CR, McCarron PO, McConville J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010;10:46. 10.1186/1471-2377-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonini G, Habetswallner F, Inghilleri M, et al. Estimation of myasthenia gravis prevalence in Italy using real-world data. J Neurol Sci. 2021. 10.1016/j.jns.2021.118340.34624796 [Google Scholar]
- 5.Evoli A, Iorio R. Controversies in ocular Myasthenia Gravis. Front Neurol. 2020;11: 605902. 10.3389/fneur.2020.605902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afifi AK, Bell WE. Tests for juvenile myasthenia gravis: comparative diagnostic yield and prediction of outcome. J Child Neurol. 1993;8:403–11. 10.1177/088307389300800422. [DOI] [PubMed] [Google Scholar]
- 7.Evoli A, Antonini G, Antozzi C, et al. Italian recommendations for the diagnosis and treatment of myasthenia gravis. Neurol Sci. 2019;40:1111–24. 10.1007/s10072-019-03746-1. [DOI] [PubMed] [Google Scholar]
- 8.Vinciguerra C, Bevilacqua L, Lupica A, et al. Diagnosis and management of seronegative Myasthenia gravis: lights and shadows. Brain Sci. 2023;13: 1286. 10.3390/brainsci13091286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baggi F, Andreetta F, Maggi L, et al. Complete stable remission and autoantibody specificity in myasthenia gravis. Neurology. 2013;80:188–95. 10.1212/WNL.0b013e31827b907b. [DOI] [PubMed] [Google Scholar]
- 10.Schneider-Gold C, Hagenacker T, Melzer N, Ruck T. Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord. 2019;12:1756286419832242. 10.1177/1756286419832242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonini G, Habetswallner F, Inghilleri M, et al. Real world study on prevalence, treatment and economic burden of myasthenia gravis in Italy. Heliyon. 2023;9: e16367. 10.1016/j.heliyon.2023.e16367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders DB, Evoli A. Immunosuppressive therapies in myasthenia gravis. Autoimmunity. 2010;43:428–35. 10.3109/08916930903518107. [DOI] [PubMed] [Google Scholar]
- 13.Mantegazza R, Bonanno S, Camera G, Antozzi C. Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr Dis Treat. 2011;7:151–60. 10.2147/NDT.S8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of myasthenia gravis. Neurol Clin. 2018;36:311–37. 10.1016/j.ncl.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice JB, White AG, Scarpati LM, et al. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39:2216–29. 10.1016/j.clinthera.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Tannemaat MR, Verschuuren JJGM. Emerging therapies for autoimmune myasthenia gravis: towards treatment without corticosteroids. Neuromuscul Disord. 2020;30:111–9. 10.1016/j.nmd.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Cioncoloni D, Casali S, Ginanneschi F, et al. Major motor-functional determinants associated with poor self-reported health-related quality of life in myasthenia gravis patients. Neurol Sci. 2016;37:717–23. 10.1007/s10072-016-2556-3. [DOI] [PubMed] [Google Scholar]
- 18.Nagane Y, Murai H, Imai T, et al. Social disadvantages associated with myasthenia gravis and its treatment: a multicentre cross-sectional study. BMJ Open. 2017;7: e013278. 10.1136/bmjopen-2016-013278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsuki M, Sugahara H, Kojima Y, et al. Comparison of quality of life and activity of daily living status of patients with myasthenia gravis treated with low-dose and high- dose prednisolone. Neuro Endocrinol Lett. 2020;41:173–8. [PubMed] [Google Scholar]
- 20.Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87:419–25. 10.1212/WNL.0000000000002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of Myasthenia gravis: 2020 update. Neurology. 2021;96:114–22. 10.1212/WNL.0000000000011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiendl H, Abicht A, Chan A, et al. Guideline for the management of myasthenic syndromes. Ther Adv Neurol Disord. 2023;16:17562864231213240. 10.1177/17562864231213240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah YS, Henderson AD, Carey AR. Effect of initial prednisone dosing on ocular Myasthenia gravis control. J Neuroophthalmol. 2021;41:e622–6. 10.1097/WNO.0000000000001058. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S, Katyal N, Narula N, Govindarajan R. Adverse side effects associated with corticosteroid therapy: a study in 39 patients with generalized Myasthenia gravis. Med Sci Monit. 2021. 10.12659/MSM.933296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma R, Wolfe GI, Kupersmith MJ. Ocular myasthenia gravis—how effective is low dose prednisone long term? J Neurol Sci. 2021;420: 117274. 10.1016/j.jns.2020.117274. [DOI] [PubMed] [Google Scholar]
- 26.Benatar M, Mcdermott MP, Sanders DB, et al. Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): a randomized, controlled trial. Muscle Nerve. 2016;53:363–9. 10.1002/mus.24769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Threetong T, Poonyathalang A, Preechawat P, et al. Initial treatment response in ocular Myasthenia gravis: a comparison between low and moderate doses of prednisolone. Clin Ophthalmol. 2020;14:2051–6. 10.2147/OPTH.S261259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuey NH. Ocular myasthenia gravis: a review and practical guide for clinicians. Clin Exp Optom. 2022;105:205–13. 10.1080/08164622.2022.2029683. [DOI] [PubMed] [Google Scholar]
- 29.Chang C-C, Yeh J-H, Chen Y-M, et al. Clinical predictors of prolonged hospital stay in patients with Myasthenia gravis: a study using machine learning algorithms. J Clin Med. 2021;10: 4393. 10.3390/jcm10194393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llabrés M, Molina-Martinez FJ, Miralles F. Dysphagia as the sole manifestation of Myasthenia gravis. J Neurol Neurosurg Psychiatry. 2005;76:1297–300. 10.1136/jnnp.2004.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in Myasthenia gravis. N Engl J Med. 2016;375:511–22. 10.1056/NEJMoa1602489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Wang M, Xu L, et al. Cancer occurrence following azathioprine treatment in myasthenia gravis patients: a systematic review and meta-analysis. Clin Neurosci. 2021;88:70–4. 10.1016/j.jocn.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Zhang C, Bu B, Yang H, et al. Immunotherapy choice and maintenance for generalized myasthenia gravis in China. CNS Neurosci Ther. 2020;26:1241–54. 10.1111/cns.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su S, Lei L, Fan Z, et al. Clinical predictors of relapse in a cohort of steroid- treated patients with well-controlled myasthenia gravis. Front Neurol. 2022;13: 816243. 10.3389/fneur.2022.816243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sghirlanzoni A, Peluchetti D, Mantegazza R, et al. Myasthenia gravis: prolonged treatment with steroids. Neurology. 1984;34:170–4. 10.1212/wnl.34.2.170. [DOI] [PubMed] [Google Scholar]
- 36.Fukui S, Nakai T, Kawaai S, et al. Advantages of an alternate-day glucocorticoid treatment strategy for the treatment of IgG4-related disease: a preliminary retrospective cohort study. Medicine (Baltimore). 2022;101: e30932. 10.1097/MD.0000000000030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.(2022) Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 14e | AccessMedicine | McGraw Hill Medical. https://accessmedicine.mhmedical.com/book.aspx?bookID=3191. Accessed 18 Jul 2022
- 38.Wilf-Yarkoni A, Lotan I, Steiner I, Hellmann MA. Chronic low-dose intravenous immunoglobulins as steroid-sparing therapy in myasthenia gravis. J Neurol. 2021;268:3871–7. 10.1007/s00415-021-10544-3. [DOI] [PubMed] [Google Scholar]
- 39.Farrugia ME, Goodfellow JA. A practical approach to managing patients with myasthenia gravis—opinions and a review of the literature. Front Neurol. 2020. 10.3389/fneur.2020.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connell K, Ramdas S, Palace J. Management of juvenile myasthenia gravis. Front Neurol. 2020;11:743. 10.3389/fneur.2020.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilhus NE. Myasthenia gravis can have consequences for pregnancy and the developing child. Front Neurol. 2020;11:554. 10.3389/fneur.2020.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piccioni MG, Tabacco S, Giannini A, et al. Myasthaenia gravis in pregnancy, delivery and newborn. Minerva Ginecol. 2020;72:30–5. 10.2373/S0026-4784.20.04505-0. [DOI] [PubMed] [Google Scholar]
- 43.Safipour Z, van der Zanden R, van den Bergh J, et al. The use of oral glucocorticoids and the risk of major osteoporotic fracture in patients with myasthenia gravis. Osteoporos Int. 2022;33:649–58. 10.1007/s00198-021-06101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park H-S, Kim K, Yu MH, et al. Risk of fracture in patients with myasthenia gravis: a nationwide cohort study in Korea. J Bone Miner Res. 2024. 10.1093/jbmr/zjae043. [DOI] [PubMed] [Google Scholar]
- 45.Lotan I, Hellmann MA, Wilf-Yarkoni A, et al. Exacerbation of myasthenia gravis following corticosteroid treatment: what is the evidence? A systematic review. J Neurol. 2021;268:4573–86. 10.1007/s00415-020-10264-0. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Li F, Zhu H, et al. Less is not necessarily more: low-dose corticosteroid therapy and long-term prognosis in generalized myasthenia gravis after thymectomy. Neurol Sci. 2022;43:3949–56. 10.1007/s10072-022-05897-0. [DOI] [PubMed] [Google Scholar]
- 47.Alhaidar MK, Abumurad S, Soliven B, Rezania K. Current treatment of Myasthenia gravis. J Clin Med. 2022;11: 1597. 10.3390/jcm11061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braga AC, Pinto C, Santos E, Braga J. Myasthenia gravis in pregnancy: experience of a Portuguese center. Muscle Nerve. 2016;54:715–20. 10.1002/mus.25095. [DOI] [PubMed] [Google Scholar]
- 49.Sharshar T, Porcher R, Demeret S, et al. Comparison of corticosteroid tapering regimens in myasthenia gravis: a randomized clinical trial. JAMA Neurol. 2021;78:426–33. 10.1001/jamaneurol.2020.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum Dis Clin North Am. 2017;43:489–502. 10.1016/j.rdc.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Díez-Porras L, Homedes C, Alberti MA, et al. Intravenous immunoglobulins may prevent prednisone-exacerbation in myasthenia gravis. Sci Rep. 2020;10:13497. 10.1038/s41598-020-70539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evoli A, Spagni G, Monte G, Damato V. Heterogeneity in myasthenia gravis: considerations for disease management. Expert Rev Clin Immunol. 2021;17:761–71. 10.1080/1744666X.2021.1936500. [DOI] [PubMed] [Google Scholar]
- 53.Katyal N, Narula N, Govindarajan R. Clinical experience with eculizumab in treatment-refractory acetylcholine receptor antibody-positive generalized myasthenia gravis. J Neuromuscul Dis. 2021;8:287–94. 10.3233/JND-200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22:383–94. 10.1016/S1474-4422(23)00077-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Q, Zhou R, Yang H, Yang H. To be or not to be vaccinated: that is a question in myasthenia gravis. Front Immunol. 2021;12: 733418. 10.3389/fimmu.2021.733418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zinman L, Thoma J, Kwong JC, et al. Safety of influenza vaccination in patients with myasthenia gravis: a population-based study. Muscle Nerve. 2009;40:947–51. 10.1002/mus.21440. [DOI] [PubMed] [Google Scholar]
- 57.Corradini P, Agrati C, Apolone G, et al. Humoral and T-cell immune response after 3 doses of messenger RNA severe acute respiratory syndrome coronavirus 2 vaccines in fragile patients: the Italian VAX4FRAIL study. Clin Infect Dis. 2023;76:e426–38. 10.1093/cid/ciac404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reyes-Leiva D, López-Contreras J, Moga E, et al. Immune response and safety of SARS-CoV-2 mRNA-1273 vaccine in patients with myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. 2022;9: e200002. 10.1212/NXI.0000000000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esposito S, Bruno C, Berardinelli A, et al. Vaccination recommendations for patients with neuromuscular disease. Vaccine. 2014;32:5893–900. 10.1016/j.vaccine.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Ou S-M, Liu C-J, Chang Y-S, et al. Tuberculosis in myasthenia gravis. Int J Tuberc Lung Dis. 2013;17:79–84. 10.5588/ijtld.12.0260. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Jiang B, Li Y, et al. A case report of disseminated nocardiosis with ocular involvement in a myasthenia gravis patient and literature review. BMC Neurol. 2019;19:243. 10.1186/s12883-019-1482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chitasombat MN, Ratchatanawin N, Visessiri Y. Disseminated extrapulmonary Legionella pneumophila infection presenting with panniculitis: case report and literature review. BMC Infect Dis. 2018;18:467. 10.1186/s12879-018-3378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prior DE, Nurre E, Roller SL, et al. Infections and the relationship to treatment in neuromuscular autoimmunity. Muscle Nerve. 2018;57:927–31. 10.1002/mus.26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strijbos E, Tannemaat MR, Alleman I, et al. A prospective, double-blind, randomized, placebo-controlled study on the efficacy and safety of influenza vaccination in myasthenia gravis. Vaccine. 2019;37:919–25. 10.1016/j.vaccine.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Ruiter AM, Strijbos E, de Meel RHP, et al. Accuracy of patient-reported data for an online patient registry of autoimmune myasthenia gravis and Lambert-Eaton myasthenic syndrome. Neuromuscul Disord. 2021;31:622–32. 10.1016/j.nmd.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Utsugisawa K, Suzuki S, Nagane Y, et al. Health-related quality-of-life and treatment targets in myasthenia gravis. Muscle Nerve. 2014;50:493–500. 10.1002/mus.24213. [DOI] [PubMed] [Google Scholar]
- 67.Konno S, Suzuki S, Masuda M, et al. Association between glucocorticoid-induced osteoporosis and myasthenia gravis: a cross-sectional study. PLoS One. 2015;10: e0126579. 10.1371/journal.pone.0126579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruce BB, Kupersmith MJ. Safety of prednisone for ocular myasthenia gravis. J Neuroophthalmol. 2012;32:212–5. 10.1097/WNO.0b013e3182536558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sussman J, Farrugia ME, Maddison P, et al. Myasthenia gravis: association of British neurologists’ management guidelines. Pract Neurol. 2015;15:199–206. 10.1136/practneurol-2015-001126. [DOI] [PubMed] [Google Scholar]
- 70.Chang C-C, Chen Y-K, Chiu H-C, Yeh J-H. Assessment of sarcopenia and obesity in patients with myasthenia gravis using dual-energy X-ray absorptiometry: a cross-sectional study. J Pers Med. 2021;11(11): 1139. 10.3390/jpm11111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eastell R, Reid DM, Compston J, et al. A UK consensus group on management of glucocorticoid-induced osteoporosis: an update. J Intern Med. 1998;244:271–92. 10.1046/j.1365-2796.1998.00408.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.