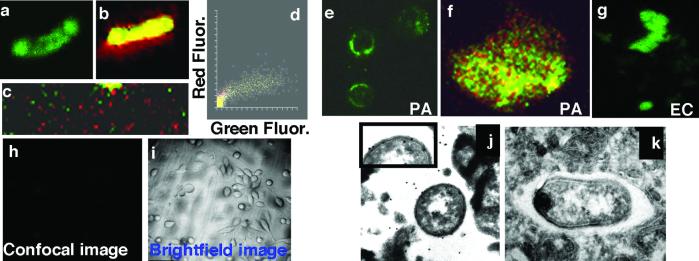
Figure 5.
Epithelial cells extract LPS from bacterial cells. (a– c) Confocal images of wt FITC-LPS reincorporated into the outer membrane of live P. aeruginosa cells. (a) FITC fluorescence (green channel) of wt FITC-LPS in the membrane of P. aeruginosa strain PAO1-V. (b) PAO1-V cells with reincorporated wt FITC-LPS were counterstained with SYTO-17 (red), and cells were visualized in both the red and green channels. (c) Low-power (×400) view of a field of PAO1-V cells with reincorporated wt-FITC-LPS. (d) Colocalization box plot showing distributions of intensity of red and green fluorescent (Fluor) stains. (e–g) Cultured wt CFTR human epithelial cells extracted LPS from live bacterial cells. (e) Wt P. aeruginosa FITC-LPS as extracted from the outer membrane of PAO1-V by epithelial cells (×400) and visualized as a diffuse, green stain. (f) High-magnification (×1,000) scan of a cell from e, showing diffuse, green LPS and punctate, red P. aeruginosa cells. (g) Wt P. aeruginosa FITC-LPS reincorporated into E. coli was extracted from the outer membrane, as visualized by the diffuse green staining. (h and i) Wt P. aeruginosa FITC-LPS reincorporated into PAO1-V cells was not extracted from the outer membrane by epithelial cells expressing ΔF508 CFTR. Confocal (h) and bright-field (i) images, as indicated (×100). (j and k) Extraction and endocytosis of P. aeruginosa LPS in vacuoles of wt CFTR epithelial cells. (j) Gold particles (20 nm) demonstrated the presence of LPS on the osmiophilic coat of ingested P. aeruginosa PAO1-V as well as in a space in close proximity to the bacterium. Inset provides an enlarged view of a portion of the bacterial surface to demonstrate LPS molecules detected by the immunogold stain. (k) Control using normal rabbit serum. (×25,000.)