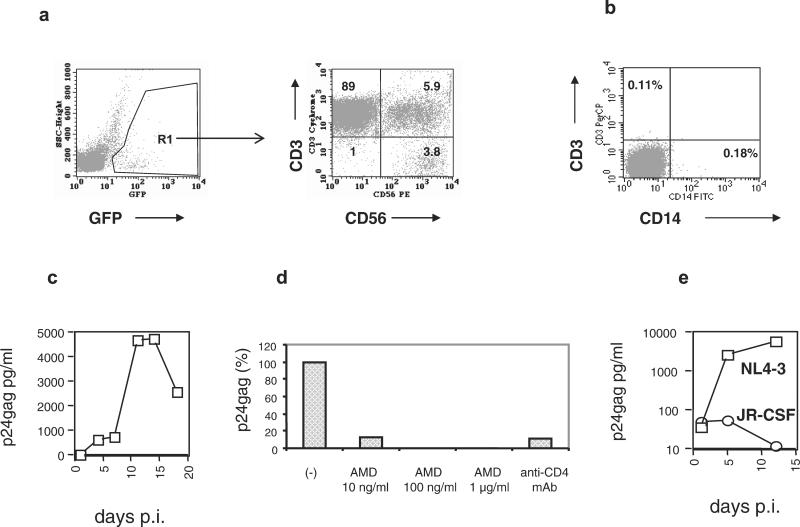
Figure 1.
In vitro infection of primary NK cells. (a) Primary PBMC were infected with GFP-tagged HIV-1 molecular clones and stained with anti-CD3 and anti-CD56 mAbs. Infected cells were gated according to the expression of GFP (Left). The phenotype of 10,000 green cells is shown (Right). Numbers within the quadrants represent the percentage of positive cells. The NK cells (CD3−CD56+) in the lower right quadrant represent 3.8% of the infected cells. The presented results were obtained at day 14 after infection. (b) Purified NK cells are free of T cells and monocyte/macrophages. After depleting monocytes and T lymphocytes from PBMC, CD56+ cells were positively selected by immunomagnetic beads and analyzed by FACS. (c) HIV-1 infection of purified human NK cells with the R5 HIV-1 strain JR-CSF at a multiplicity of infection of 0.1. Similar results were obtained in an additional experiment. (d) Anti-CD4 mAbs and the CXCR4-specific antiviral compound AMD3100 inhibit HIV-1 entry into NK cells. Purified primary NK cells were incubated with 5 μg of an anti-CD4 HIV-neutralizing antibody or different concentrations of AMD3100 for 30 min before exposure to the X4 HIV-1 strain NL4–3. After 1 h, the cells were washed and cultured in the absence of anti-CD4 antibodies. AMD3100 was present in the culture medium throughout the entire experiment. (e) NK cells from a blood donor homozygous for the Δ32 CCR5 mutation were purified and exposed to NL4–3 (X4) and JR-CSF (R5) HIV-1 strains. Viral replication was monitored by p24gag determinations in culture supernatants.