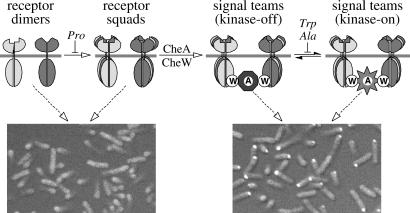
Figure 5.
Model of chemoreceptor cluster assembly and organization. Receptors of different detection specificities (indicated by shading differences) can associate, most likely through their common trimer contact residues, to form mixed receptor squads, which then bind CheA and CheW to form collaborative signaling teams. Component stoichiometries in the receptor complexes are not yet known, but squads and signal teams may be based on trimers of dimers. Proline mutations at the trimer contact sites of Tsr generally block squad formation, whereas alanine and tryptophan mutations allow assembly of signal teams that are locked in the kinase-on or kinase-off state. The connections between signaling teams that lead to macroscopic cluster formation are not explicitly shown. The light micrographs at the bottom of the figure show examples of the two extreme receptor-clustering phenotypes seen in this work. Mutant receptors unable to form signal teams, e.g., proline mutants, failed to assemble clusters detectable with a YFP-CheZ reporter (Left, Tsr-I377P). Mutant receptors able to assemble signal teams, regardless of their ability to respond to serine stimuli, e.g., alanine and tryptophan mutants, formed clusters (Right, Tsr-I377W).