Abstract
Background
We previously showed increased cortical grey matter (GM) volume in CogEx trial participants who performed cognitive rehabilitation (CR). Here, we explore combined CR and aerobic exercise (EX) effects on regional changes in brain volumes and white matter (WM) integrity.
Methods
Seventy-three patients were randomized into four groups receiving a combination of CR and EX or their sham versions: CR + EX, CR + EX-sham, EX + CR-sham, and CR-sham + EX-sham. A diagnosis of progressive multiple sclerosis (PMS) and impaired information processing speed were required for inclusion. Participants attended a 12-week intervention twice/week. Assessments were performed at baseline, week-12 (W12), and nine months post-baseline (M9). Structural MRI scans were acquired with a standardized protocol, and voxelwise variations of brain volumes and WM fractional anisotropy (FA) were analyzed.
Results
Baseline regional brain volumes and WM FA were comparable between groups. Voxelwise analyses at W12 and M9 revealed generalized volume reductions in all groups. We found different patterns of volumetric changes in the left inferior temporal gyrus between CR + EX and CR-sham + EX-sham, and in the right cerebellum crus II between EX + CR-sham and CR + EX-sham. WM FA values remained stable throughout the trial and no longitudinal between-group differences were found.
Conclusions
Our analysis showed a decrease in brain volumes and limited effects of the combined CR + EX intervention, indicating that the previously found cortical GM increase was not superimposable at voxel level. Methodological and sampling differences between the studies could explain these discrepancies. In few cognitively relevant areas, the combined CR interventions might have affected patterns of volume changes, while EX modified cerebellar motor regions.
Clinical trial registration
The main trial was registered on ClinicalTrials.gov (NCT03679468; registration date: 20 Sep 2018).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-025-13382-9.
Keywords: Multiple Sclerosis, Magnetic Resonance Imaging, Exercise Therapy, Cognitive Rehabilitation, Voxelwise, Neuroplasticity
Introduction
Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease affecting the central nervous system with heterogeneous clinical manifestations. Cognitive impairment is highly prevalent in people with MS, and the proportion of affected patients is greater in progressive phenotypes [1]. Compared with cognitively preserved patients, those with any degree of cognitive impairment tend to be older, have MS for more years, and present more widespread structural damage, in terms both of focal white matter (WM) lesions and atrophy [2]. These alterations were found to be more pronounced in patients with more severe deficits or with an involvement of multiple cognitive domains [2, 3]. In recent years, the possibility of improving cognitive dysfunction with cognitive rehabilitation and physical exercise and exploring the response of the central nervous system to such stimuli has gained interest. The combination of these approaches has been studied mainly in relapsing–remitting MS, while effects in patients with progressive MS (PMS) and greater disease severity have not been investigated systematically [4].
The CogEx study [5] assessed whether there is a synergistic effect on cognitive functioning when both cognitive rehabilitation (CR) and aerobic exercise (EX) are administered to patients with PMS and impaired processing speed. This was expected to contribute to a better understanding of the application of both interventions in a subgroup where impaired brain plasticity and reserve could represent limiting factors [6, 7].
The analyses conducted up to now after the end of the trial [5, 8] showed that performing CR and EX in combination did not improve processing speed any better than single or sham interventions. However, a clinically meaningful cognitive improvement, defined as an increase of more than 4 points on the Symbol Digit Modalities Test (SDMT) following treatment, was observed in a substantial proportion of patients, regardless of the intervention type, with 60% of all participants demonstrating this improvement [5]. On the other hand, the analysis of structural and functional magnetic resonance imaging (MRI) data highlighted differential effects between treatments, with CR resulting in increased global cortical grey matter (GM) volume and increased activity of several areas during a cognitive task [8]. We also showed that a significant increase of cortical GM volume was observed in frontal, parietal, and temporal lobes, indicating that intervention effects might be specific to brain regions that were more involved during training [8].
In this context, voxelwise MRI analyses can be employed to better characterize localized structural modifications in brain GM and WM. These methodologies have been used to assess neurodegeneration in MS [9–11], which is more pronounced and widespread in patients with PMS [12], and to study neuroplastic adaptations following rehabilitation [13, 14]. Potential structural changes following rehabilitation have been hypothesized to occur as a result of angiogenesis, dendrite pruning, remyelination, decrease of inflammation level and consequent change of the microstructure [15, 16]. Whether these effects are localized and whether they are persistent once rehabilitation is finished is uncertain. It is also unknown how these mechanisms interact with the progressive neurodegenerative damage that characterizes people with PMS.
To address these points, we describe the explorative analysis of GM and WM modifications at voxel level within the CogEx MRI substudy both in volume and in microstructure. Our objective was to evaluate the effect of the combined CR and EX intervention, as well as the single treatment components, on these outcomes, and to study whether regional structural neuroplasticity represents a possible substrate of the changes in cognitive performance observed in the whole sample. We hypothesized that structural adaptations would be evident in cortical regions/WM tracts connected to the stimuli provided during treatment, and that, given the aim for which the CogEx trial was designed, the most prominent associations with cognitive Changes after treatment would be found in the combined CR and EX group. Considering previous reports showing Changes in both WM integrity and brain volumes after 12 weeks of CR and EX (alone or combined) in a healthy aging sample, we assumed this timeframe to be appropriate also for detecting structural neuroplasticity in our study [17, 18].
Materials and methods
Study design and participants
The CogEx trial was a randomized sham-controlled trial. After screening, the baseline assessment was performed, and patients were randomized to one of four treatment arms (1:1:1:1 ratio): CR + EX; CR + EX-sham; EX + CR-sham and CR-sham + EX-sham. Then, they underwent 12 weeks of intervention, twice per week, and performed follow-up assessments at the end of the intervention (W12) and nine months from the baseline assessment (M9). Of the 11 centres involved in the CogEx trial, four participated in the MRI substudy: (a) IRCCS San Raffaele Hospital (Milan, Italy); (b) University of Genoa (Genoa, Italy); (c) University of Alabama at Birmingham (Birmingham, Alabama, USA) and d) Kessler Foundation (East Hanover, New Jersey, USA).
Patients were enrolled between 14th Dec 2018 and 2nd April 2022. Complete inclusion and exclusion criteria of the CogEx trial are reported elsewhere [5, 19]. Importantly, patients were required to have a confirmed diagnosis of PMS, age between 25 and 65 years, an Expanded Disability Status Scale (EDSS) score lower than 7.0, and impaired information processing speed according to their performance in the SDMT (below the 10th percentile of published normative data in each country). Participants were excluded if they performed habitual aerobic exercise, had undergone treatment with steroids in the 3 months prior and had a history of substance abuse or severe mental illness. Additionally, as specific criteria for the analyses performed in the current study, participants were required to have complete neuropsychological and structural MRI assessments at all three timepoints, with sufficient image quality in either T1-weighted or diffusion-weighted MRI scans.
Interventions
Full details regarding the interventions, including information on duration, content, modality, and progression of each treatment component have been reported extensively in the appendix of the main publication [5]. Briefly, CR was provided with the computerized RehaCom program using modules of divided and sustained attention, concentration, and vigilance. CR-sham consisted of Internet training with durations of personnel contact and computer usage matched with the CR group. EX consisted of aerobic exercise performed on a recumbent arm-leg step ergometer (NuStep T5XR, Ann Arbor, MI, USA), alternating each session between moderate-intensity continuous training and high-intensity interval training. EX-sham was focused on balance training and stretching, designed specifically to avoid any cardiovascular effort and any cognitive-motor dual tasking. While the duration of CR and CR-sham sessions was fixed at around 40 min, the duration of EX and EX-sham session increased progressively throughout the trial from 20 to 60 min. After the end of the 12 weeks of intervention no additional treatment, apart from the usual care, was provided.
MRI outcomes
Acquisition protocol
Using 3.0 Tesla scanners (IRCCS San Raffaele: Philips Ingenia CX; University of Genoa and University of Alabama: Siemens Prisma; Kessler Foundation: Siemens Skyra) and standardized guidelines for participants’ positioning, the following brain MRI sequences were acquired: a) variable flip angle 3D T2-weighted fluid-attenuated inversion recovery (FLAIR) turbo spin echo (Philips scanner: repetition time [TR] = 4800 ms; echo time [TE] = 270 ms; inversion time [TI] = 1650 ms; matrix size = 256 × 256; field of view [FOV] = 256 × 256 mm2; echo train length [ETL] = 167; 192 contiguous sagittal slices, 1 mm thick; Siemens scanners: TR = 5000 ms; TE = 395 ms; TI = 1800 ms; matrix size = 256 × 256; FOV = 256 × 256 mm2; ETL = 284; 192 contiguous sagittal slices, 1.05 mm thick), b) sagittal 3D T1-weighted sequence: (Philips scanner: TR = 7 ms; TE = 3.2 ms; TI = 1000 ms; flip angle = 8°; matrix size = 256 × 256; FOV = 256 × 256 mm2; 204 contiguous sagittal slices, 1 mm thick; Siemens scanners: TR = 2300 ms; TE = 2.98 ms; TI = 900 ms; flip angle = 9°; matrix size = 256 × 256; FOV = 256 × 256 mm2; 204 contiguous sagittal slices, 1 mm thick); and c) axial pulsed-gradient spin echo single shot diffusion-weighted echo planar imaging (EPI) (all scanners: 3 shells at b-value = 700/1000/2855 s/mm2 along 6/30/60 non-collinear directions and 10 b = 0 volumes were acquired, FOV = 240 × 233 mm, pixel size = 2.14 × 2.69 mm, 56 slices, 2.3 mm-thick, matrix = 112 × 85, TR = about 6000 ms, TE = about 80 ms and three additional b = 0 volumes with reversed polarity of gradients for distortion correction).
Conventional MRI analysis
T2-hyperintense lesions were identified on baseline 3D FLAIR scans using an automated segmentation approach and their volume (LV) was obtained. Normalized brain, GM and WM volumes (NBV, NGMV and NWMV) were extracted from lesion-filled 3D T1-weighted scans at baseline. Detailed processing steps are described elsewhere [8].
Voxel-based and tensor-based morphometry
Tensor-based morphometry (TBM), as implemented in SPM12, was used to map changes of regional brain volumes over time. Longitudinal registration was used to align each patients’ lesion-filled scans to a mid-point average template [20], which was then used for iterative groupwise alignment using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) method [21]. Finally, an affine transformation that maps from the population average (DARTEL Template space) to Montreal Neurological Institute (MNI) space was calculated, the rate of longitudinal volume changes (difference of jacobians of the deformation) were spatially normalized to MNI and smoothed with an 8 mm Gaussian kernel.
The steps described for groupwise alignment were repeated for baseline 3D T1-weighted images to run a voxel-based morphometry (VBM) analysis. The only difference in the procedure described above is that normalization to MNI space was applied to brain maps.
Tract-based spatial statistics
Preprocessing of diffusion-weighted imaging data included correction for off-resonance and eddy current induced distortions, using the Eddy tool within the FSL library [22].
The diffusion tensor (DT) was estimated in each voxel using the shell at b ≤ 1000 s/mm2 by linear regression [23] using the FMRIB’s Diffusion Toolbox.
A longitudinal pipeline free of interpolation asymmetries was applied [24] using the spatial normalization methods [25] supported by the DTI-TK toolkit: first an unbiased within-subject template was generated from all the DT volumes of each patient, which was then used to produce a study specific template [26]. Fractional anisotropy (FA) maps from the population specific DTI template and from the transformed individual DTI were derived. Finally, a tract-based spatial statistics (TBSS) analysis [27] was used to perform a voxelwise analysis of whole-brain WM FA. In detail, the population FA template was thinned to create a WM tract “skeleton”, which was thresholded at FA > 0.2 to include only WM voxels. Individual-subject FA values were projected onto this group skeleton by searching perpendicular from the skeleton for maximum FA value.
For statistical analysis of differences between changes at W12 vs baseline and changes at M9 vs W12, skeletonized FA values of the earlier time-point were subtracted from those of the subsequent one (“W12—baseline” and “M9—W12”).
Clinical and neuropsychological outcomes
Clinical and neuropsychological assessments were performed at baseline, W12, and M9 by assessors blinded to treatment allocation.
Cognitive performances were assessed with the Brief International Cognitive Assessment of Multiple Sclerosis (BICAMS), a reliable and sensitive measure of cognition in people with MS [28], which includes the SDMT for processing speed evaluation, the Brief Visuospatial Memory Test Revised (BVMT-R) for visual memory evaluation and the California Verbal Learning Test-II (CVLT-II) for verbal memory evaluation. Age-, sex-, and education-adjusted z-scores for cognitive tests were computed according to country-specific normative values.
Expanded Disability Status Scale (EDSS) scores were provided by each participant’s treating neurologist at baseline only. Also, evaluations of walking capacity (6-min walking test), physical activity and cardio-respiratory fitness were performed at all time points.
Complete information regarding the methodology of each evaluation is reported in the protocol paper [19].
Statistical analysis
Statistical analysis was performed using SPSS (IBM, version 26.0) for demographic and clinical data, SPM12 for voxelwise volumetric data, and FSL randomise for voxelwise diffusivity data.
Descriptive statistics were reported as means (standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables, while categorical variables were reported as frequencies. Between-group comparisons were performed using Chi-square, ANOVA (with Bonferroni-corrected post-hoc tests), and Kruskal–Wallis tests as appropriate.
Volumetric and diffusivity Changes were assessed within and between groups using one-way ANOVAs with a 4-levels factor adjusting for acquisition center. All comparisons were corrected for age and sex. For TBSS analysis, a permutation-based inference for non-parametric statistical thresholding was used, with number of permutations = 5000 and threshold-free cluster enhancement (TFCE) applied. For comparisons between changes at W12 vs baseline and changes at M9 vs W12, correction for follow-up length was applied. Additionally, in the TBM model a 2-level within-group factor for period was added, whereas for TBSS the difference between FA changes at W12 vs baseline and changes at M9 vs W12 was computed and analyzed. All comparisons were examined at the FWE-corrected threshold (p < 0.05) and at the uncorrected threshold (p < 0.001), with a cluster extent threshold of 10 voxels. Sensitivity analyses grouping CR vs CR-sham and EX vs EX-sham were also performed for all between-group comparisons.
Age- and sex-corrected multiple linear regression models were used to assess correlations between longitudinal volumetric/FA changes and changes in cognitive outcomes.
Results
Sample characteristics
A total of 73 patients with valid MRI and neuropsychological data at all time points were included in the current analysis. Due to the presence of movement artifacts some scans were not usable. Thus, 72 patients were included in the TBM analysis and 68 in the TBSS analysis.
Demographic, clinical, neuropsychological and conventional MRI baseline characteristics are reported in Table 1. There were significant differences between groups in CVLT z-score, which was higher in CR-sham + EX-sham than CR + EX-sham (p = 0.05), and in NBV, which was lower in CR + EX-sham than CR-sham + EX-sham (p = 0.002).
Table 1.
Main demographic, clinical, neuropsychological and conventional MRI characteristics at baseline of multiple sclerosis (MS) patients included in this study, divided according to treatment allocation
| CR + EX | CR + EX-sham | EX + CR-sham | CR-sham + EX-sham | P | |
|---|---|---|---|---|---|
| N | 18 | 20 | 18 | 17 | |
| Participants from Centers: San Raffaele/Genoa/Alabama/Kessler [N] | 8/8/2/0 | 9/6/3/2 | 8/8/1/1 | 6/7/4/0 | 0.70+ |
| Mean age [years] (SD) | 50.4 (8.8) | 52.7 (6.5) | 52.5 (6.0) | 52.2 (7.0) | 0.77* |
| Sex (M/F) | 9/9 | 7/13 | 6/12 | 4/13 | 0.43+ |
| Median EDSS score (IQR) | 5.25 (4.5–6.0) | 5.25 (4.25–6.25) | 5.75 (4.0–6.5) | 6.0 (4.5–6.5) | 0.71++ |
| Mean disease duration [years] (SD) | 12.7 (11.0) | 17.0 (9.0) | 15.4 (11.5) | 19.8 (10.0) | 0.23* |
| Type of MS (Primary/Secondary progressive) | 7/11 | 4/16 | 4/14 | 2/15 | 0.28+ |
| Mean 6MWT total distance [m] (SD) | 232.1 (142.0) | 256.6 (109.7) | 224.8 (116.2) | 282.2 (145.6) | 0.77* |
| Mean VO2peak [ml/min/kg] (SD) | 15.1 (5.4) | 16.7 (6.6) | 16.0 (4.6) | 14.0 (6.5) | 0.55* |
| Mean WRpeak [W] (SD) | 73.3 (26.6) | 76.0 (31.3) | 74.1 (26.5) | 75.0 (36.5) | 0.99* |
| Mean average % in MVPA (SD) | 1.7 (1.9) | 1.4 (2.0) | 2.2 (3.5) | 1.4 (1.5) | 0.74* |
| Mean education [total years of schooling] (SD) | 12.2 (3.7) | 13.9 (3.5) | 14.1 (2.9) | 14.6 (3.6) | 0.17* |
| SDMT – mean number of correct responses (SD) | 29.8 (7.4) | 32.5 (6.2) | 30.5 (5.9) | 34.0 (9.2) | 0.31* |
| Mean SDMT z-score (SD) | – 1.94 (0.5) | – 1.97 (0.7) | – 2.02 (0.6) | – 1.83 (0.4) | 0.78* |
| Mean CVLT-II z-score (SD) | – 1.18 (0.9) | – 1.41 (1.0) | – 1.23 (1.0) | – 0.50 (1.1) | 0.04* |
| Mean BVMT-R z-score (SD) | – 0.38 (0.9) | – 0.52 (1.4) | – 0.35 (1.1) | – 0.26 (0.7) | 0.90* |
| Mean T2 LV [ml] (SD) | 9.4 (8.9) | 12.5 (10.9) | 17.3 (11.5) | 8.2 (8.9) | 0.11* |
| Mean NBV [ml] (SD) | 1485 (63) | 1439 (52) | 1471 (62) | 1512 (60) | 0.004* |
| Mean NGMV [ml] (SD) | 815 (45) | 792 (53) | 803 (48) | 833 (30) | 0.05* |
| Mean NWMV [ml] (SD) | 670 (34) | 647 (25) | 668 (43) | 679 (44) | 0.06* |
Significant differences (p < 0.05) are highlighted in bold*ANOVA model; +Chi-square test, ++Kruskall-Wallis test
Clinical and neuropsychological outcomes
Full details regarding the longitudinal analysis of clinical and neuropsychological outcomes are reported in the main publication [5].
Briefly, there were no differences between groups regarding cognitive functions after treatment, although 171 (60%) of the 284 participants analyzed showed an improvement of at least 4 points on the SDMT at W12. Regarding aerobic fitness, there were significant improvements at W12 in EX versus EX-sham groups, which were not maintained at M9. No differences were observed in walking capacity or physical activity measures.
Regional Volumetric analysis
At baseline, there were no regional volumetric differences between the four treatment arms (FWE-corrected threshold).
Longitudinal differences – W12 vs baseline
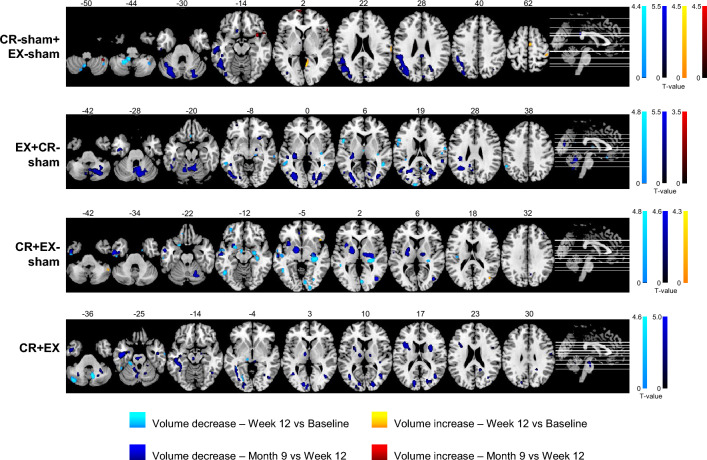
Within-groups volumetric changes from baseline to W12 showed a few clusters of increased volume and several clusters of decreased volume in occipital, temporal, frontal, parietal and cerebellar areas in all groups (uncorrected threshold).
Longitudinal volumetric changes from baseline to W12 were not significantly different between the four treatment arms (FWE-corrected threshold).
At the uncorrected threshold, there were significant effects of treatment on right lingual gyrus volume (increased in CR-sham + EX-sham compared with CR + EX-sham and CR + EX), left cerebellum lobule IX volume (decreased in CR-sham + EX-sham and CR + EX compared with EX + CR-sham), and right cerebellum lobule VIII volume (decreased in CR + EX compared with EX + CR-sham and CR-sham + EX-sham).
Detailed findings from this analysis are reported in Tables 2 and 3.
Table 2.
Within-group longitudinal volumetric Changes at week 12 vs baseline
| Comparison | kE | pFWE | T | MNIx | MNIy | MNIz | BA | Area |
|---|---|---|---|---|---|---|---|---|
| CR-sham + EX-sham increase | 72 | 0.282 | 4.49 | 8 | – 56 | 2 | 18 | R Lingual Gyrus |
| 10 | 0.905 | 3.79 | 66 | – 21 | 21 | 22 | R SMG | |
| 11 | 0.94 | 3.71 | 66 | – 16 | 20 | 48 | R SMG | |
| 18 | 0.947 | 3.7 | 45 | – 33 | 62 | 3 | R Postcentral Gyrus | |
| 14 | 0.982 | 3.57 | – 18 | – 22 | – 24 | – | L PHG | |
| 23 | 0.998 | 3.39 | 4 | – 9 | 63 | 6 | R SMA | |
| CR-sham + EX-sham decrease | 256 | 0.331 | 4.43 | – 9 | – 42 | – 44 | – | L Cerebellum Lobule IX |
| 99 | 0.839 | 3.88 | – 15 | – 68 | – 56 | – | L Cerebellum Lobule VIII | |
| 44 | 0.933 | 3.73 | 39 | – 52 | – 46 | – | R Cerebellum Lobules VIIb | |
| 44 | 0.972 | 3.62 | – 6 | – 58 | – 51 | – | L Cerebellum Lobule IX | |
| 11 | 0.999 | 3.33 | – 44 | 12 | – 38 | 20 | L ITG | |
| EX + CR-sham increase | – | |||||||
| EX + CR-sham decrease | 236 | 0.12 | 4.81 | – 57 | – 44 | – 3 | 21 | L MTG |
| 137 | 0.302 | 4.46 | – 14 | – 96 | 22 | 18 | L SOG | |
| 263 | 0.563 | 4.17 | – 54 | 14 | 18 | 44 | L IFG Pars Opercularis | |
| 124 | 0.78 | 3.95 | 48 | – 44 | 6 | 21 | R MTG | |
| 53 | 0.783 | 3.95 | – 54 | – 51 | 38 | 40 | L IPG | |
| 14 | 0.942 | 3.71 | 16 | – 22 | – 10 | – | R Hippocampus | |
| 64 | 0.951 | 3.69 | – 36 | – 34 | 15 | 48 | L Rolandic Operculum | |
| 19 | 0.96 | 3.66 | 54 | – 20 | 21 | 48 | R Rolandic Operculum | |
| 47 | 0.966 | 3.64 | – 56 | 4 | 16 | 48 | L Precentral Gyrus | |
| 10 | 0.974 | 3.61 | – 2 | – 12 | 8 | – | L Thalamus | |
| 18 | 0.976 | 3.6 | – 18 | – 75 | – 57 | – | L Cerebellum Lobule VIII | |
| 14 | 0.976 | 3.6 | 3 | – 54 | 16 | 30 | R Precuneus | |
| 26 | 0.981 | 3.58 | 63 | – 6 | 18 | 43 | R Postcentral Gyrus | |
| 36 | 0.987 | 3.54 | 34 | – 20 | 3 | 48 | R Insula | |
| 16 | 0.987 | 3.54 | 62 | – 26 | – 10 | 20 | R MTG | |
| 14 | 0.999 | 3.36 | – 2 | 24 | – 18 | 11 | L Gyrus Rectus | |
| CR + EX-sham increase | 27 | 0.471 | 4.27 | 48 | – 50 | – 45 | – | R Cerebellum Crus II |
| 19 | 0.901 | 3.79 | 46 | 22 | – 4 | – | R IFG Pars Orbitalis | |
| 10 | 0.998 | 3.37 | 44 | – 70 | 16 | 39 | R MTG | |
| CR + EX-sham decrease | 894 | 0.108 | 4.84 | 28 | – 28 | 2 | – | R Hippocampus |
| 167 | 0.184 | 4.66 | 6 | – 81 | 0 | 17 | R Lingual Gyrus | |
| 160 | 0.298 | 4.47 | – 20 | 10 | – 24 | – | L Inferior OFC | |
| 224 | 0.333 | 4.42 | – 46 | – 54 | – 8 | 37 | L ITG | |
| 116 | 0.695 | 4.04 | – 58 | – 3 | – 10 | 22 | L MTG | |
| 129 | 0.752 | 3.98 | – 44 | – 2 | – 48 | – | L ITG | |
| 25 | 0.891 | 3.81 | – 39 | – 18 | 20 | 48 | L Rolandic Operculum | |
| 72 | 0.912 | 3.77 | – 33 | 48 | – 15 | 11 | L IFG Pars Orbitalis | |
| 89 | 0.92 | 3.76 | – 50 | – 14 | – 8 | 48 | L MTG | |
| 36 | 0.976 | 3.6 | 22 | – 64 | 26 | 18 | R Cuneus | |
| 53 | 0.985 | 3.55 | – 14 | 56 | – 18 | 11 | L Superior OFC | |
| 59 | 0.988 | 3.53 | 21 | – 94 | – 4 | 18 | R IOG | |
| 30 | 0.991 | 3.5 | – 12 | 39 | – 27 | 11 | L Superior OFC | |
| 13 | 0.992 | 3.49 | 20 | 8 | – 16 | - | R Amygdala | |
| 11 | 0.993 | 3.48 | – 63 | – 16 | – 6 | 22 | L MTG | |
| 15 | 0.994 | 3.46 | 30 | – 75 | – 4 | 19 | R Fusiform Gyrus | |
| 23 | 0.997 | 3.42 | – 60 | – 21 | – 26 | 20 | L ITG | |
| CR + EX increase | – | |||||||
| CR + EX decrease | 243 | 0.215 | 4.6 | 8 | – 62 | – 34 | – | R Cerebellum Lobule VIII |
| 229 | 0.545 | 4.19 | – 40 | – 72 | – 39 | – | L Cerebellum Crus II | |
| 42 | 0.976 | 3.6 | – 26 | – 30 | – 26 | – | L Cerebellum Lobules IV/V | |
| 17 | 0.977 | 3.6 | 63 | – 9 | – 28 | 21 | R ITG | |
| 21 | 0.981 | 3.58 | – 9 | – 66 | – 45 | – | L Cerebellum Lobule VIII | |
| 18 | 0.988 | 3.53 | – 26 | – 68 | – 3 | 19 | L Lingual Gyrus | |
| 33 | 0.991 | 3.5 | – 45 | – 44 | – 27 | 37 | L ITG | |
| 16 | 0.999 | 3.34 | – 15 | – 40 | – 44 | – | L Cerebellum Lobule IX |
Results analyzed at cluster extent threshold = 10 voxels, p < 0.001 uncorrected and p < 0.05 FWE-corrected
BA Brodmann Area, CR Cognitive rehabilitation, CR-sham Sham cognitive rehabilitation, EX Aerobic exercise, EX-sham Sham exercise, IFG Inferior Frontal Gyrus, IOG Inferior Occipital Gyrus, IPG Inferior Parietal Gyrus, ITG Inferior Temporal Gyrus, kE Cluster extent, L Left, MTG Middle Temporal Gyrus, OFC Orbitofrontal Cortex, PHG Parahippocampal Gyrus, R Right, SMA Supplementary Motor Area, SMG Supramarginal Gyrus, SOG Superior Occipital Gyrus
Table 3.
Between-group longitudinal volumetric Changes at week 12 vs baseline
| Comparison | kE | pFWE | F/T | MNIx | MNIy | MNIz | BA | Area |
|---|---|---|---|---|---|---|---|---|
| Group effect | 18 | 0.908 | 8.17 | 8 | – 54 | 3 | 10 | R Lingual Gyrus |
| 25 | 0.918 | 8.12 | – 15 | – 50 | – 42 | – | L Cerebellum Lobule IX | |
| 43 | 0.998 | 7.03 | 10 | – 70 | – 36 | – | R Cerebellum Lobule VIII | |
| EX + CR-sham > CR-sham + EX-sham | 91 | 0.453 | 4.29 | – 15 | – 50 | – 44 | – | L Cerebellum Lobule IX |
| EX + CR-sham < CR-sham + EX-sham | 52 | 0.665 | 4.07 | 66 | – 14 | 18 | 22 | R Postcentral Gyrus |
| 24 | 0.956 | 3.67 | – 54 | – 50 | 38 | 40 | L IPG | |
| 16 | 0.963 | 3.65 | – 2 | – 14 | 8 | – | L Thalamus | |
| 15 | 0.983 | 3.56 | 66 | – 22 | 22 | 2 | R SMG | |
| 13 | 0.999 | 3.32 | 24 | – 74 | – 12 | 18 | R Lingual Gyrus | |
| CR + EX-sham > CR-sham + EX-sham | 24 | 0.994 | 3.47 | 46 | – 50 | – 46 | – | R Cerebellum Crus II |
| CR + EX-sham < CR-sham + EX-sham | 60 | 0.199 | 4.63 | 8 | – 54 | 3 | 18 | R Lingual Gyrus |
| 346 | 0.73 | 4.01 | 34 | – 22 | – 6 | – | R Hippocampus | |
| 29 | 0.988 | 3.53 | – 12 | 34 | – 26 | 11 | L Superior OFC | |
| CR + EX > CR-sham + EX-sham | – | |||||||
| CR + EX < CR-sham + EX-sham | 76 | 0.817 | 3.91 | – 26 | – 28 | – 27 | 30 | L Fusiform Gyrus |
| 16 | 0.94 | 3.71 | – 46 | – 46 | – 27 | 37 | L ITG | |
| 12 | 0.96 | 3.66 | 8 | – 56 | 3 | 18 | R Lingual Gyrus | |
| 24 | 0.994 | 3.46 | 10 | – 64 | – 38 | – | R Cerebellum Lobule VIII | |
| CR + EX-sham > EX + CR-sham | 150 | 0.574 | 4.16 | -52 | 12 | 18 | 44 | L IFG Pars Opercularis |
| 125 | 0.82 | 3.91 | 63 | – 14 | 18 | 48 | R Postcentral Gyrus | |
| 90 | 0.972 | 3.62 | 42 | – 68 | 15 | 37 | R MTG | |
| 17 | 0.99 | 3.51 | – 14 | – 96 | 22 | 18 | L SOG | |
| CR + EX-sham < EX + CR-sham | 19 | 0.984 | 3.56 | 26 | – 96 | – 4 | 18 | R Calcarine Sulcus |
| CR + EX > EX + CR-sham | 15 | 0.896 | 3.8 | – 54 | 14 | 18 | 44 | L IFG Pars Opercularis |
| 53 | 0.98 | 3.58 | – 14 | – 96 | 24 | 18 | L SOG | |
| CR + EX < EX + CR-sham | 312 | 0.308 | 4.46 | 10 | – 70 | – 36 | – | R Cerebellum Lobule VIII |
| 54 | 0.941 | 3.71 | – 14 | – 52 | – 40 | – | L Cerebellum Lobule IX | |
| 12 | 0.983 | 3.57 | – 18 | – 45 | – 18 | – | L Cerebellum Lobules IV/V | |
| 22 | 0.996 | 3.43 | – 39 | – 72 | – 40 | – | L Cerebellum Crus II | |
| CR + EX > CR + EX-sham | – | |||||||
| CR + EX < CR + EX-sham | 32 | 0.748 | 3.99 | 63 | – 10 | – 28 | 20 | R ITG |
| 110 | 0.824 | 3.9 | – 24 | – 33 | – 26 | – | L Cerebellum Lobules IV/V | |
| EX > EX-sham | – | |||||||
| EX < EX-sham | 93 | 0.44 | 4.3 | 64 | – 15 | 18 | – | R Postcentral Gyrus |
| 16 | 0.893 | 3.81 | 6 | 66 | – 16 | 11 | R Superior OFC | |
| 43 | 0.974 | 3.61 | – 26 | – 30 | – 26 | – | L Cerebellum Lobules IV/V | |
| 41 | 0.978 | 3.59 | 57 | – 8 | 21 | 43 | R Postcentral Gyrus | |
| CR > CR-sham | 61 | 0.974 | 3.61 | 46 | – 70 | 33 | 39 | R Angular Gyrus |
| 10 | 0.999 | 3.31 | – 14 | – 98 | 22 | 18 | L SOG | |
| CR < CR-sham | 42 | 0.345 | 4.41 | 8 | – 54 | 2 | 18 | R Lingual Gyrus |
| 173 | 0.713 | 4.02 | 9 | – 62 | – 34 | – | R Cerebellum Lobule VIII | |
| 18 | 0.987 | 3.54 | – 20 | – 48 | – 16 | – | L Cerebellum Lobules IV/V |
Results analyzed at cluster extent threshold = 10 voxels, p < 0.001 uncorrected and p < 0.05 FWE-corrected
BA Brodmann Area, CR Cognitive rehabilitation, CR-sham Sham cognitive rehabilitation, EX Aerobic exercise, EX-sham Sham exercise, IFG Inferior Frontal Gyrus, IPG Inferior Parietal Gyrus, ITG Inferior Temporal Gyrus, kE Cluster extent, L Left, MTG Middle Temporal Gyrus, OFC Orbitofrontal Cortex, R Right, SMG Supramarginal Gyrus, SOG Superior Occipital Gyrus
There were no significant correlations between longitudinal volumetric changes from baseline to W12 and changes in cognitive performances (FWE-corrected threshold). Correlations significant at the uncorrected threshold are reported in Supplementary Table 1.
Longitudinal within-group volumetric changes from baseline to W12 and from W12 to M9 are shown in Fig. 1.
Fig. 1.
Within-group volume decreases and increases from baseline to W12 and from W12 to M9 in the four treatment groups (p < 0.001, uncorrected, cluster extent threshold = 10 voxels). Significant clusters at the two time points were overlaid on the ch2better template in MRIcron (https://www.nitrc.org/projects/mricron) and axial slices, with the corresponding MNI z coordinate shown on top, were extracted. Images are presented in neurological convention
Longitudinal differences – M9 vs W12
Within-group volumetric changes from W12 to M9 showed several clusters of decreased volume in occipital, temporal, cerebellar and subcortical areas in all groups (FWE-corrected and uncorrected thresholds).
Longitudinal volumetric changes from W12 to M9 were not significantly different between the four treatment arms (FWE-corrected threshold).
At the uncorrected threshold, there was no significant effect of treatment.
Detailed findings from this analysis are reported in Tables 4 and 5.
Table 4.
Within-group longitudinal volumetric Changes at month 9 vs week 12
| Comparison | kE | pFWE | T | MNIx | MNIy | MNIz | BA | Area |
|---|---|---|---|---|---|---|---|---|
| CR-sham + EX-sham increase | 13 | 0.225 | 4.51 | 16 | 6 | – 32 | 28 | R PHG |
| 44 | 0.38 | 4.29 | 51 | 20 | – 16 | 38 | R STP | |
| 44 | 0.485 | 4.18 | -8 | 69 | 3 | 10 | L Medial SFG | |
| 58 | 0.493 | 4.17 | 36 | 14 | – 16 | 38 | R Insula | |
| 19 | 0.677 | 3.98 | 56 | 24 | 0 | 45 | R IFG Pars Triangularis | |
| 35 | 0.722 | 3.94 | 38 | – 44 | – 50 | – | R Cerebellum Lobule VIII | |
| 10 | 0.794 | 3.86 | 52 | 14 | – 3 | – | R Insula | |
| CR-sham + EX-sham decrease | 1032 | 0.011 | 5.51 | – 30 | – 72 | – 27 | – | L Cerebellum Crus I |
| 5416 | 0.112 | 4.76 | – 44 | – 69 | 18 | 39 | L MOG | |
| 406 | 0.227 | 4.51 | 27 | – 82 | – 32 | - | R Cerebellum Crus I | |
| 475 | 0.331 | 4.35 | – 57 | – 21 | – 10 | 20 | L MTG | |
| 34 | 0.52 | 4.14 | 15 | – 75 | 14 | 18 | R Calcarine Sulcus | |
| 350 | 0.522 | 4.14 | 24 | – 69 | 32 | 19 | R SOG | |
| 241 | 0.617 | 4.05 | 15 | – 90 | – 4 | 18 | R Lingual Gyrus | |
| 70 | 0.741 | 3.92 | 0 | – 36 | 27 | 23 | R PCC | |
| 36 | 0.787 | 3.87 | – 9 | -8 | 6 | – | L Thalamus | |
| 60 | 0.895 | 3.72 | – 34 | 16 | 12 | 48 | L Insula | |
| 70 | 0.921 | 3.67 | – 54 | – 3 | – 9 | 22 | L STG | |
| 46 | 0.93 | 3.65 | 4 | – 84 | 26 | 18 | R Cuneus | |
| 20 | 0.958 | 3.58 | – 45 | – 34 | – 15 | 20 | L ITG | |
| 60 | 0.963 | 3.57 | – 9 | 24 | – 16 | 11 | L Gyrus Rectus | |
| 23 | 0.967 | 3.55 | – 51 | – 21 | – 32 | 20 | L ITG | |
| 20 | 0.977 | 3.51 | 34 | – 81 | 10 | 19 | R MOG | |
| 26 | 0.982 | 3.48 | 16 | – 74 | 54 | 7 | R SPG | |
| 13 | 0.983 | 3.48 | 24 | – 84 | 34 | 19 | R SOG | |
| 21 | 0.988 | 3.44 | 16 | – 84 | 15 | 19 | R Calcarine Sulcus | |
| 18 | 0.993 | 3.4 | 9 | – 56 | 14 | 30 | R Calcarine Sulcus | |
| EX + CR-sham increase | 11 | 0.979 | 3.5 | 66 | – 4 | 20 | 43 | R Postcentral Gyrus |
| EX + CR-sham decrease | 3287 | 0.012 | 5.48 | 4 | – 56 | – 26 | – | Cerebellar Vermis VIII |
| 419 | 0.015 | 5.4 | – 32 | – 28 | 2 | – | L Hippocampus | |
| 3588 | 0.021 | 5.3 | – 36 | – 72 | 0 | 19 | L MOG | |
| 194 | 0.197 | 4.56 | – 16 | – 62 | – 18 | – | L Cerebellum Lobule VI | |
| 1679 | 0.327 | 4.36 | 24 | – 58 | 16 | 17 | R Calcarine Sulcus | |
| 115 | 0.372 | 4.3 | 56 | – 38 | 18 | 42 | R STG | |
| 152 | 0.551 | 4.11 | 3 | – 82 | 12 | 18 | L Calcarine Sulcus | |
| 215 | 0.652 | 4.01 | – 45 | – 12 | – 27 | 20 | L ITG | |
| 93 | 0.733 | 3.93 | – 8 | – 69 | – 44 | – | L Cerebellum Lobule VIII | |
| 139 | 0.76 | 3.9 | 26 | 18 | – 10 | 48 | R Insula | |
| 50 | 0.875 | 3.75 | – 30 | 51 | – 10 | 11 | L Middle OFC | |
| 185 | 0.904 | 3.7 | – 14 | – 50 | 30 | – | L Precuneus | |
| 25 | 0.936 | 3.64 | 16 | – 48 | 21 | – | R Precuneus | |
| 51 | 0.945 | 3.62 | – 50 | – 27 | – 2 | 21 | L MTG | |
| 41 | 0.955 | 3.59 | – 22 | – 100 | 4 | 17 | L MOG | |
| 28 | 0.96 | 3.58 | 9 | -42 | 9 | 29 | R Precuneus | |
| 30 | 0.972 | 3.53 | 28 | 56 | – 12 | 11 | R Middle OFC | |
| 30 | 0.978 | 3.51 | – 15 | – 46 | – 21 | – | L Cerebellum Lobules IV/V | |
| 19 | 0.985 | 3.47 | 10 | – 64 | – 58 | – | R Cerebellum Lobule VIII | |
| 14 | 0.99 | 3.43 | – 62 | – 51 | 10 | 21 | L MTG | |
| 17 | 0.996 | 3.35 | – 20 | – 76 | 24 | 19 | L SOG | |
| 11 | 0.996 | 3.35 | – 14 | – 72 | 18 | 18 | L Calcarine Sulcus | |
| 15 | 0.998 | 3.28 | – 24 | – 60 | – 33 | – | L Cerebellum Lobule VI | |
| CR + EX-sham increase | – | |||||||
| CR + EX-sham decrease | 1062 | 0.193 | 4.57 | – 9 | – 2 | – 6 | – | L Pallidum |
| 924 | 0.231 | 4.5 | – 52 | – 8 | – 34 | 20 | L ITG | |
| 188 | 0.241 | 4.48 | 42 | – 74 | 3 | 19 | R MOG | |
| 687 | 0.313 | 4.38 | 32 | – 14 | – 2 | - | R Putamen | |
| 366 | 0.7 | 3.96 | – 33 | 12 | 0 | 48 | L Insula | |
| 30 | 0.785 | 3.87 | 8 | – 63 | 36 | 7 | R Precuneus | |
| 235 | 0.808 | 3.84 | 44 | 39 | 27 | 45 | R IFG Pars Triangularis | |
| 235 | 0.847 | 3.79 | 24 | – 68 | – 26 | – | R Cerebellum Lobule VI | |
| 41 | 0.936 | 3.64 | – 27 | – 72 | – 9 | 18 | L Fusiform Gyrus | |
| 23 | 0.977 | 3.51 | – 6 | 39 | – 6 | 11 | L ACC | |
| 10 | 0.98 | 3.5 | – 58 | – 9 | 8 | 22 | L STG | |
| 23 | 0.991 | 3.42 | 58 | – 9 | – 18 | 21 | R MTG | |
| CR + EX increase | – | |||||||
| CR + EX decrease | 2349 | 0.051 | 5.02 | – 45 | – 14 | – 24 | 20 | L ITG |
| 744 | 0.288 | 4.41 | – 15 | – 86 | 4 | 17 | L SOG | |
| 540 | 0.387 | 4.29 | 39 | – 64 | 16 | 39 | R MTG | |
| 165 | 0.573 | 4.09 | – 10 | – 9 | 6 | – | L Thalamus | |
| 451 | 0.605 | 4.06 | 18 | – 84 | 9 | 18 | R Calcarine Sulcus | |
| 611 | 0.698 | 3.96 | – 39 | 15 | 18 | 48 | L IFG Pars Opercularis | |
| 150 | 0.744 | 3.91 | 45 | – 6 | – 30 | 20 | R ITG | |
| 327 | 0.812 | 3.84 | – 6 | – 66 | – 27 | – | L Cerebellum Lobule VI | |
| 148 | 0.856 | 3.78 | – 24 | – 52 | – 30 | – | L Cerebellum Lobule VIII | |
| 104 | 0.877 | 3.75 | 15 | – 50 | 24 | 23 | R Precuneus | |
| 142 | 0.899 | 3.71 | 34 | – 58 | – 32 | – | R Cerebellum Crus I | |
| 210 | 0.927 | 3.66 | 9 | – 56 | – 32 | – | R Cerebellum Lobule VIII | |
| 43 | 0.938 | 3.64 | – 16 | – 81 | 33 | 18 | L SOG | |
| 35 | 0.954 | 3.6 | – 52 | – 51 | 40 | 40 | L IPG | |
| 59 | 0.967 | 3.55 | 21 | – 38 | – 28 | – | R Cerebellum Lobules I/IV | |
| 32 | 0.973 | 3.53 | 57 | – 10 | 32 | 43 | R Postcentral Gyrus | |
| 11 | 0.986 | 3.46 | – 22 | – 62 | 0 | 19 | L Lingual Gyrus | |
| 20 | 0.987 | 3.45 | 14 | – 12 | 9 | – | R Thalamus | |
| 23 | 0.988 | 3.45 | 46 | 6 | 26 | 44 | R IFG Pars Opercularis | |
| 14 | 0.988 | 3.45 | 15 | – 9 | – 14 | – | R Hippocampus | |
| 20 | 0.989 | 3.44 | – 30 | – 51 | – 44 | – | L Cerebellum Lobule VIII | |
| 36 | 0.991 | 3.42 | 44 | – 57 | – 15 | 37 | R ITG | |
| 10 | 0.996 | 3.35 | 30 | – 63 | – 3 | 19 | R Fusiform Gyrus |
Results analyzed at cluster extent threshold = 10 voxels, p < 0.001 uncorrected and p < 0.05 FWE-corrected
ACC Anterior Cingulate Cortex, BA Brodmann Area, CR Cognitive rehabilitation, CR-sham Sham cognitive rehabilitation, EX Aerobic exercise, EX-sham Sham exercise, IFG Inferior Frontal Gyrus, IOG Inferior Occipital Gyrus, IPG Inferior Parietal Gyrus, ITG Inferior Temporal Gyrus, kE Cluster extent, L Left, MFG Middle Frontal Gyrus, MOG Middle Occipital Gyrus, MTG Middle Temporal Gyrus, OFC Orbitofrontal Cortex, PCC Posterior Cingulate Cortex, PHG Parahippocampal Gyrus, R Right, SFG Superior Frontal Gyrus, SOG Superior Occipital Gyrus, SPG Superior Parietal Gyrus, STG Superior Temporal Gyrus, STP Superior Temporal Pole
Table 5.
Between-group longitudinal volumetric Changes at month 9 vs week 12
| Comparison | kE | pFWE | T | MNIx | MNIy | MNIz | BA | Area |
|---|---|---|---|---|---|---|---|---|
| EX + CR-sham > CR-sham + EX-sham | 10 | 0.995 | 3.36 | – 10 | 22 | – 21 | 11 | L Gyrus Rectus |
| EX + CR-sham < CR-sham + EX-sham | 60 | 0.596 | 4.07 | 56 | – 36 | 20 | 42 | R STG |
| 22 | 0.953 | 3.6 | – 8 | – 68 | -46 | – | L Cerebellum Lobule VIII | |
| 10 | 0.988 | 3.45 | 6 | – 58 | – 36 | – | Cerebellar Vermis IX | |
| 22 | 0.993 | 3.4 | 30 | 57 | – 12 | 11 | R Middle OFC | |
| 27 | 0.994 | 3.39 | 33 | – 51 | – 48 | – | R Cerebellum Lobule VIII | |
| CR + EX-sham > CR-sham + EX-sham | – | |||||||
| CR + EX-sham < CR-sham + EX-sham | 43 | 0.84 | 3.8 | 38 | 16 | – 14 | 38 | R Insula |
| 42 | 0.859 | 3.78 | 52 | 24 | 30 | 44 | R IFG Pars Triangularis | |
| 15 | 0.997 | 3.32 | 39 | – 63 | – 54 | – | R Cerebellum Lobule VIII | |
| CR + EX > CR-sham + EX-sham | 28 | 0.833 | 3.81 | – 58 | – 3 | 0 | 48 | L STG |
| CR + EX < CR-sham + EX-sham | 71 | 0.897 | 3.72 | 56 | – 9 | 28 | 43 | R Postcentral Gyrus |
| 10 | 0.994 | 3.38 | 38 | – 58 | – 58 | – | R Cerebellum Lobule VIII | |
| CR + EX-sham > EX + CR-sham | 204 | 0.343 | 4.34 | 8 | – 74 | – 30 | – | R Cerebellum Crus II |
| 33 | 0.978 | 3.51 | – 51 | – 66 | 8 | 37 | L MTG | |
| CR + EX-sham < EX + CR-sham | 38 | 0.941 | 3.63 | 39 | 40 | 34 | 46 | R MFG |
| 12 | 0.986 | 3.46 | – 52 | 12 | – 22 | 38 | L MTG | |
| CR + EX > EX + CR-sham | – | |||||||
| CR + EX < EX + CR-sham | – | |||||||
| CR + EX > CR + EX-sham | 101 | 0.701 | 3.96 | – 38 | 6 | 0 | 48 | L Insula |
| 19 | 0.895 | 3.72 | – 58 | – 8 | 6 | 48 | L STG | |
| 18 | 0.978 | 3.51 | 42 | 51 | 16 | 46 | R MFG | |
| 25 | 0.988 | 3.45 | – 60 | – 18 | – 8 | 21 | L MTG | |
| CR + EX < CR + EX-sham | – | |||||||
| EX > EX-sham | 18 | 0.955 | 3.59 | – 45 | 21 | – 4 | 47 | L IFG Pars Orbitalis |
| EX < EX-sham | 261 | 0.503 | 4.16 | 30 | – 48 | – 42 | – | R Cerebellum Lobule VIII |
| 33 | 0.892 | 3.73 | – 8 | – 68 | – 46 | – | L Cerebellum Lobule VIII | |
| 39 | 0.944 | 3.62 | – 34 | – 28 | 0 | – | L Hippocampus | |
| 25 | 0.967 | 3.55 | 15 | 32 | – 14 | 11 | R Superior OFC | |
| 33 | 0.969 | 3.54 | 12 | 50 | – 16 | 11 | R Gyrus Rectus | |
| 17 | 0.982 | 3.49 | 9 | – 57 | – 33 | – | R Cerebellum Lobule VIII | |
| 12 | 0.994 | 3.38 | 8 | – 74 | – 28 | – | R Cerebellum Crus II | |
| CR > CR-sham | 49 | 0.967 | 3.55 | – 46 | – 75 | – 16 | 19 | L IOG |
| 30 | 0.988 | 3.44 | – 51 | – 60 | – 18 | 37 | L ITG | |
| CR < CR-sham | 10 | 0.956 | 3.59 | 52 | 26 | 28 | 45 | R IFG Pars Triangularis |
| 16 | 0.993 | 3.4 | 51 | 9 | 26 | 44 | R IFG Pars Opercularis |
Results analyzed at cluster extent threshold = 10 voxels, p < 0.001 uncorrected and p < 0.05 FWE-corrected
BA Brodmann Area, CR Cognitive rehabilitation, CR-sham Sham cognitive rehabilitation, EX Aerobic exercise, EX-sham Sham exercise, IFG Inferior Frontal Gyrus, IOG Inferior Occipital Gyrus, ITG Inferior Temporal Gyrus, kE Cluster extent, L Left, MFG Middle Frontal Gyrus, MTG Middle Temporal Gyrus, OFC Orbitofrontal Cortex, R Right, STG Superior Temporal Gyrus
There were no significant correlations between longitudinal volumetric changes from W12 to M9 and changes in cognitive performances (FWE-corrected threshold). Correlations significant at the uncorrected threshold are reported in Supplementary Table 2.
Longitudinal differences – M9 changes vs W12 changes
There was a significant difference in longitudinal volumetric changes at M9 vs W12 and changes at W12 vs baseline between the four treatment arms (FWE-corrected threshold and significant group-by-time interaction). In particular, there were significantly different patterns of change in left inferior temporal gyrus volume between CR + EX and CR-sham + EX-sham, where volume decreased from baseline to W12 and was stable from W12 to M9 in CR + EX, and was stable from baseline to W12 and decreased from W12 to M9 in CR-sham + EX-sham. Also, there were significantly different patterns of change in right cerebellum crus II volume between EX + CR-sham and CR + EX-sham, which was stable from baseline to W12 in EX + CR-sham and decreased from W12 to M9 in CR + EX-sham, where volume decreased at W12 and was stable at M9. Figure 2 shows results of the group-by-time interaction as assessed with SPM12.
Fig. 2.
Volumetric longitudinal changes in the four treatment groups: comparison between changes at M9 vs W12 and changes at W12 vs baseline (p < 0.001, uncorrected, cluster extent threshold = 10 voxels). A Significant clusters are shown on the left side, projected onto a glass brain. The design matrix shown on the right side contains eight cells representing longitudinal changes in the four groups at W12 vs baseline (W12-BL) and at M9 vs W12 (M9-W12), and the seven additional covariates (age, sex, four dummy variables for the acquisition centers, and follow-up length). Above the design matrix, the structure of the F-contrast (group-by-time interaction, as computed in SPM12) is reported. B Significant clusters overlaid on the customised grey matter template image. C Demeaned and adjusted group effects at peak-level of the significant cluster in Cerebellum crus II/lobule VIII are plotted (grey line). The different behavior between EX + CR-sham and CR + EX-sham can be observed. D The same plot for the significant cluster in the inferior temporal gyrus is shown. Here, the different behavior between CR-sham + EX-sham and CR + EX can be observed
Detailed findings from this analysis are reported in Table 6.
Table 6.
Between-group longitudinal volumetric differences in the Changes at month 9 vs week 12 and Changes at week 12 vs baseline
| Comparison | kE | pFWE | F/T | MNIx | MNIy | MNIz | BA | Area |
|---|---|---|---|---|---|---|---|---|
| Group by time interaction | 93 | 0.705 | 7.97 | 8 | – 74 | – 30 | – | R Cerebellum Crus II |
| 29 | 0.762 | 7.82 | – 45 | – 45 | – 27 | – | L ITG | |
| EX + CR-sham > CR-sham + EX-sham | 74 | 0.943 | 3.54 | – 16 | – 52 | – 42 | – | L Cerebellum Lobule IX |
| 19 | 0.987 | 3.39 | 6 | – 72 | – 42 | – | R Cerebellum Lobule VIIb | |
| 20 | 0.996 | 3.29 | 34 | – 54 | – 48 | – | R Cerebellum Lobule VIII | |
| EX + CR-sham < CR-sham + EX-sham | 32 | 0.716 | 3.83 | – 52 | 34 | – 6 | – | L IFG Pars Orbitalis |
| 36 | 0.789 | 3.76 | 22 | – 90 | 32 | 18 | R SOG | |
| 99 | 0.932 | 3.57 | – 4 | 26 | – 18 | 11 | L Gyrus Rectus | |
| 42 | 0.966 | 3.48 | – 50 | 16 | 3 | 45 | L IFG Pars Triangularis | |
| 28 | 0.971 | 3.47 | 12 | – 82 | – 14 | – | R Cerebellum Lobule VI | |
| 23 | 0.976 | 3.44 | 64 | – 4 | 18 | 43 | R Postcentral Gyrus | |
| 14 | 0.994 | 3.32 | – 2 | – 12 | 6 | – | L Thalamus | |
| CR + EX-sham > CR-sham + EX-sham | 45 | 0.846 | 3.7 | 48 | – 75 | 6 | 19 | R MOG |
| 43 | 0.945 | 3.54 | 40 | – 62 | – 54 | – | R Cerebellum Lobule VIIb | |
| 32 | 0.972 | 3.46 | – 33 | – 63 | – 62 | – | L Cerebellum Lobule VIII | |
| CR + EX-sham < CR-sham + EX-sham | 728 | 0.422 | 4.1 | – 54 | – 60 | – 20 | 37 | L ITG |
| 21 | 0.481 | 4.05 | – 24 | 60 | – 15 | 11 | L Middle OFC | |
| 120 | 0.774 | 3.78 | – 34 | – 64 | 32 | 19 | L MOG | |
| 38 | 0.9 | 3.62 | – 3 | – 15 | 8 | – | L Thalamus | |
| 36 | 0.991 | 3.36 | – 28 | – 87 | – 40 | – | L Cerebellum Crus II | |
| CR + EX > CR-sham + EX-sham | 72 | 0.734 | 3.82 | 38 | – 48 | – 48 | – | R Cerebellum Lobule VIII |
| CR + EX < CR-sham + EX-sham | 75 | 0.053 | 4.78 | – 44 | – 42 | – 28 | 37 | L ITG |
| 11 | 0.965 | 3.49 | – 20 | – 22 | – 26 | – | L PHG | |
| 10 | 0.997 | 3.28 | – 36 | – 76 | – 39 | – | L Cerebellum Crus II | |
| CR + EX-sham > EX + CR-sham | 67 | 0.952 | 3.52 | -52 | 10 | 8 | 48 | L IFG Pars Opercularis |
| 32 | 0.97 | 3.47 | 64 | – 4 | 18 | 43 | R Postcentral Gyrus | |
| 19 | 0.976 | 3.45 | 46 | – 75 | 0 | 19 | R IOG | |
| 32 | 0.989 | 3.37 | 45 | – 70 | 33 | 39 | R Angular Gyrus | |
| CR + EX-sham < EX + CR-sham | 407 | 0.04 | 4.86 | 8 | – 72 | – 30 | – | R Cerebellum Crus II |
| 23 | 0.979 | 3.43 | – 46 | – 75 | – 15 | 19 | L IOG | |
| 18 | 0.989 | 3.37 | – 14 | – 60 | – 40 | – | L Cerebellum Lobule VIII | |
| 28 | 0.996 | 3.29 | – 50 | – 66 | – 8 | 37 | L ITG | |
| CR + EX > EX + CR-sham | 13 | 0.996 | 3.3 | 44 | – 69 | 33 | 39 | R Angular Gyrus |
| CR + EX < EX + CR-sham | 15 | 0.922 | 3.59 | – 6 | – 34 | – 15 | – | L Cerebellum Lobule III |
| 82 | 0.937 | 3.56 | 8 | – 60 | – 36 | – | R Cerebellum Lobule VIII | |
| 10 | 0.998 | 3.26 | – 36 | – 75 | – 40 | – | L Cerebellum Crus II | |
| CR + EX > CR + EX-sham | – | |||||||
| CR + EX < CR + EX-sham | – | |||||||
| EX > EX-sham | 46 | 0.872 | 3.66 | 6 | – 74 | – 27 | – | Cerebellar Vermis VII |
| 15 | 0.996 | 3.29 | 34 | – 50 | – 46 | – | R Cerebellum Lobule VIII | |
| EX < EX-sham | 21 | 0.906 | 3.61 | 66 | – 16 | 20 | 48 | R SMG |
| 28 | 0.987 | 3.39 | – 14 | 21 | – 18 | 11 | L Superior OFC | |
| 17 | 0.995 | 3.31 | – 56 | 30 | 4 | 45 | L IFG Pars Triangularis | |
| CR > CR-sham | 30 | 0.969 | 3.47 | 48 | – 74 | 6 | 19 | R MOG |
| 42 | 0.971 | 3.46 | 45 | – 69 | 32 | 39 | R Angular Gyrus | |
| CR < CR-sham | 100 | 0.47 | 4.06 | – 44 | – 44 | – 27 | – | L ITG |
| 52 | 0.589 | 3.95 | 8 | – 94 | – 9 | – | R Lingual Gyrus | |
| 124 | 0.641 | 3.9 | – 20 | – 45 | – 16 | – | L Cerebellum Lobules IV/V | |
| 87 | 0.813 | 3.74 | – 46 | – 74 | – 15 | 19 | L IOG | |
| 113 | 0.831 | 3.72 | 9 | – 70 | – 33 | – | R Cerebellum Lobule VIII | |
| 62 | 0.962 | 3.5 | – 45 | – 70 | – 42 | – | L Cerebellum Crus II |
Results analyzed at cluster extent threshold = 10 voxels, p < 0.001 uncorrected and p < 0.05 FWE-corrected
BA Brodmann Area, CR Cognitive rehabilitation, CR-sham Sham cognitive rehabilitation, EX Aerobic exercise, EX-sham Sham exercise, IFG Inferior Frontal Gyrus, IOG Inferior Occipital Gyrus, ITG Inferior Temporal Gyrus, kE Cluster extent, L Left, MOG Middle Occipital Gyrus, OFC Orbitofrontal Cortex, PHG Parahippocampal Gyrus, R Right, SMG Supramarginal Gyrus, SOG Superior Occipital Gyrus
Diffusivity analysis
There were no within-group longitudinal changes and no between-group differences in WM FA in any of the four treatment arms (FWE-corrected and uncorrected thresholds).
Discussion
The analysis of volumetric modifications after treatment did not highlight clear effects of the interventions. In fact, we found a mixed pattern of volume increase and decrease in several areas in all groups. In general, only a few differences between groups were found, all not surviving the corrected threshold and only partially specific to a single group or intervention. In comparison, the effect of the disease, characterized by volume reductions in several areas (Fig. 1), was evident in all groups. This was even more pronounced in data collected 6 months after treatment, where intervention effects were extremely limited, showing only a small number of sub-threshold differences between groups, while significant progression of atrophy, representing the natural course of structural damage characterizing PMS patients [29], was present in all groups.
These observations led us to hypothesize that, due to the effect of MS-related atrophy, our initial analysis might not have been able to highlight subtler differences between interventions. To uncover possible treatment effects that previously went unnoticed, we contrasted changes observed after treatment to those seen after follow-up/observation, as there are studies showing changes in the first week of training, which tend to disappear two months after the termination of training [30]. We reasoned that, given the small number of patients included in each group, there could be high variability between groups, which could be mitigated by assessing intervention effects as a difference in the rate of volumetric changes between the two periods. This analysis also made it possible to differentiate immediate from delayed effects of the intervention and to characterize whether observed differences were due to enlargement of brain areas or a reduction in volume loss (i.e., neuroprotective effect).
The results of this analysis showed significantly different rates of volume change between the four treatment groups in the right cerebellum crus II and in the left inferior temporal cortex. In particular, left inferior temporal gyrus volume was stable during treatment and decreased after the follow-up in CR-sham + EX-sham, while the opposite pattern was observed in CR + EX, where it decreased after treatment and remained stable at follow-up. The inferior temporal gyrus, along with other structures of this lobe, is involved in semantic memory [31, 32] and changes in this region were also reported in a trial involving patients with MS, where five weeks of CR increased its functional activity during the performance of a memory task [33]. Regarding right cerebellum crus II volume, we found that it remained stable after treatment and decreased at follow-up in EX + CR-sham, while the opposite pattern of change was observed in CR + EX-sham, where volume in this region decreased during treatment and was stable at follow-up. This result might be tied to the repetitive stepping motion performed during aerobic exercise in the EX + CR-sham group, in fact this area has been demonstrated to contribute to the accurate temporal prediction of absolute timing, which is linked to the controlled repetition of a motor action [34]. Additionally, this region is part of the second non-motor representation of the cerebellum and has been shown to be involved in cognitive, emotional and social tasks [35], taking part in both language processing and working memory. Considering that both this difference and the change observed in the inferior temporal gyrus were significant in the comparison between groups that performed CR versus CR-sham, it could be possible that the CR component of the intervention is the primary driver of these modifications. However, the absence of significant correlations with improvements in cognitive functions makes it difficult to ascribe such meaning to our findings. Indeed, they could also be explained by heterogeneous atrophy dynamics between patients and a limited number of participants per group included.
We observed no effects of either CR, EX or their combination on DTI measures of WM integrity. Previous studies on this topic have found mixed results, however most included patients with relatively low disability [36]. The only study involving patients with PMS and high disability found no effects of aerobic training on WM microstructure, measured with graph metrics of structural connectivity [37]. This might indicate that WM structural plasticity in these patients is severely limited, possibly due to a depletion of their reserve after many years of disease. It is also possible that 12 weeks of training were not sufficient to impact WM microstructure, and longer treatment durations might show different results. However, longitudinal assessments in people with MS have highlighted a decline in measures of WM integrity over time, depending on the duration of follow-up and on the methodology employed for data analysis [38–40]. In the present study, the stability of FA values found in contrast to the progression of atrophy may also suggest a stabilizing effect of the administered training, similarly to the neuroprotective effects observed after treatment with some pharmacological therapies [41, 42]. Lastly, the method applied for this analysis has been optimized to improve the quality of the superposition between anatomically corresponding fiber bundles, in order to facilitate the possibility of detecting training-induced changes. Nonetheless, we cannot exclude the fact that some changes could have happened but not exactly at the same voxel level in all participants, and thus they might not have been captured in our analysis.
Compared with the results reported in the previously published analysis of global brain volumes and task-related fMRI activity from the CogEx MRI substudy [8]—where patients who underwent CR exhibited increased cortical GM volume after 12 weeks of training—the findings of the present study do not reveal substantial volume increases. One possible explanation for this difference is that the global volume increase observed previously may not correspond to superimposable local variations at the voxel-based level, considering also that the current analysis was not confined to specific tissues or regions, but assessed the whole brain. In fact, the findings from the previous study could suggest that global and lobar increases in cortical GM volume outweighed the more widespread trend of general decrease, resulting in a net positive effect. In addition, it is important to consider that the previous study included 84 patients in the analysis of W12 data (see study flowchart [8]), of which 12 had to be excluded from the current volumetric analysis, given that only patients having complete assessments at all three time points were suitable for TBM. These exclusions additionally resulted in a baseline imbalance between groups in CVLT and, more importantly, NBV, which could have further contributed to the observed discrepancies between the findings of the two studies. Considering the results of the main CogEx trial [5], the fact that the improvement observed in the main outcome was not different between the four treatment groups might indicate that there are no specific neural substrates underlying these changes. However, similar behaviors between the two groups of patients who underwent CR were observed in both of the MRI analyses performed so far, so there might be common mechanisms at play.
There are some limitations to this work. Despite the robustness of the methodology for MRI data analysis, the small number of patients with a complete assessment in each treatment group could have introduced a high degree of variability in the longitudinal changes observed at voxel level. Also, considering the extensive damage and limited capacity for structural improvements due to a depletion of brain reserves typically observed in PMS patients [7], performing an MRI scan before the baseline visit would have given us a reference to assess disease effects on neurodegeneration in each patient and to better disentangle the effects of the intervention. Lastly, findings on structural adaptations after rehabilitation in MS are still quite heterogeneous, as evidenced by a recent review [36]. While 12 weeks of treatment might be deemed sufficient in this context, based on results in healthy aging subjects [17, 18], longer treatment durations or higher intensities of training might be needed to observe more consistent effects in PMS patients, also considering the limitations outlined above.
In conclusion, the included cohort of cognitively impaired patients with PMS displayed no differences between treatment groups in localized volumetric or diffusivity changes. A trend of volume decrease in several cortical regions, likely following the natural trajectory of PMS-related neurodegeneration, was observed in all groups over the trial period. In contrast, WM FA remained generally stable, indicative of a possible neuroprotective effect. We can hypothesize that CR combined with either EX or EX-sham might result in volumetric changes of areas relevant for cognitive functions, while EX might support structural changes in motor-related cerebellar regions. However, due to the absence of relevant correlations with cognitive performance improvements other works are needed to confirm these findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Francesco Romanò contributed to data collection, analysis and interpretation of the data and drafting/revising the manuscript. Maria A. Rocca contributed to study concept, analysis and interpretation of data, and to drafting/revising the manuscript. She also acted as study supervisor. Elisabetta Pagani contributed to analysis and interpretation of data, and to drafting/revising the manuscript. Maria Pia Amato contributed to study concept, data collection and drafting/revising the manuscript. Giampaolo Brichetto contributed to study concept, data collection and drafting/revising the manuscript. Jeremy Chataway contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Nancy D. Chiaravalloti contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Gary Cutter contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Ulrik Dalgas contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. John DeLuca contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Rachel Farrel contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Peter Feys contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Jennifer Freeman contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Matilde Inglese contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Emilio Cipriano contributed to data collection and drafting/revising the manuscript. Cecilia Meza contributed to data collection and drafting/revising the manuscript. Robert W. Motl contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Amber Salter contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Brian M. Sandroff contributed to study concept, data collection and interpretation, and drafting/revising the manuscript. Anthony Feinstein contributed to study concept, data interpretation, and drafting/revising the manuscript. He also acted as study supervisor. Massimo Filippi contributed to study concept, data interpretation, and drafting/revising the manuscript. Guarantors: Maria A. Rocca and Massimo Filippi.
Funding
This study was supported in part by funds from MSCanada (grant EGID 3185). Ancillary funding was provided by the Consortium of Multiple Sclerosis Centres, the Danish Multiple Sclerosis Society, and the US National Multiple Sclerosis Society.
Data availability
Anonymized data are available one year after publication, upon reasonable request. Please make the request to the corresponding author. A CogEx Committee will review the request for approval. A data sharing agreement will be produced before any data is shared.
Declarations
Conflicts of interest
Francesco Romanò has nothing to disclose. Maria A. Rocca received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from AstraZaneca, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Horizon Therapeutics Italy, Merck Serono SpA, Novartis, Roche, Sanofi and Teva. She receives research support from the MS Society of Canada, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders; and Associate Co-Editor for Europe and Africa for Multiple Sclerosis Journal. Elisabetta Pagani has nothing to disclose. Maria Pia Amato received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, and Teva Pharmaceutical Industries, and receives research support from Biogen Idec, Merck-Serono, Roche, Pharmaceutical Industries and Fondazione Italiana Sclerosi Multipla. Giampaolo Brichetto has been awarded and receives research support from Roche, Fondazione Italiana Sclerosi Multipla, ARSEP, H2020 EU Call. In the last 3 years, Jeremy Chataway has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society and the Rosetrees Trust, he is supported in part by the NIHR University College London Hospitals (UCLH) Biomedical Research Centre, London, UK, he has been a local principal investigator for a trial in MS funded by the Canadian MS society, a local principal investigator for commercial trials funded by: Ionis, Novartis and Roche, and has taken part in advisory boards/consultancy for Azadyne, Biogen, Lucid, Janssen, Merck, NervGen, Novartis and Roche. Nancy D. Chiaravalloti is on an Advisory Board for Akili Interactive and is a member of the Editorial Boards of Multiple Sclerosis Journal and Frontiers in NeuroTrauma. Gary Cutter is a member of Data and Safety Monitoring Boards for Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Mapi Pharmaceuticals LTD, Merck, Merck/Pfizer, Opko Biologics, OncoImmune, Neurim, Novartis, Ophazyme, Sanofi Aventis, Reata Pharmaceuticals, Teva pharmaceuticals, VielaBio Inc, Vivus, NHLBI (Protocol Review Committee), NICHD (OPRU oversight committee), he is on Consulting or Advisory Boards for Biodelivery Sciences International, Biogen, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Neurogenesis LTD, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Roche, TG Therapeutics, he is employed by the University of Alabama at Birmingham and President of Pythagoras, Inc, a private consulting company located in Birmingham AL. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck-Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. John DeLuca is an Associate Editor of the Archives of Physical Medicine and Rehabilitation, and Neuropsychology Review, received compensation for consulting services and/or speaking activities from Biogen Idec, Celgene, MedRhythms, and Novartis, and receives research support from Biogen Idec, National Multiple Sclerosis Society, Consortium of Multiple Sclerosis Centers, and National Institutes of Health. Rachel Farrel has received honoraria and served on advisory panels for Merck, TEVA, Novartis, Genzyme, GW pharma (Jazz pharmaceuticals), Allergan, Merz, Ipsen and Biogen, she is supported in part by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre, London, UK. Peter Feys is editorial board member of NNR, MSJ and Frontiers in Rehabilitation Sciences (section ‘Strengthening Health Systems’), provides consultancy to NeuroCompass and was board of advisory board meetings for BIOGEN. Jennifer Freeman has been awarded research grants from the NIHR, UK. Matilde Inglese is Co-Editor for Controversies for Multiple Sclerosis Journal, received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, and received research support from NIH, NMSS, the MS Society of Canada, the Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, H2020 EU Call. Emilio Cipriano has nothing to disclose. Cecilia Meza has nothing to disclose. Robert W. Motl has nothing to disclose. Amber Salter receives research funding from Multiple Sclerosis Society of Canada, National Multiple Sclerosis Society, CMSC and the US Department of Defense, and is a member of editorial board for Neurology, and serves as a consultant for Gryphon Bio, LLC, Sora Neuroscience and Abata Therapeutics. She has equity in Owl Therapeutics. Brian M. Sandroff has nothing to disclose. Anthony Feinstein is on Advisory Boards for Akili Interactive and Roche, and reports grants from the MS Society of Canada, book royalties from Johns Hopkins University Press, Cambridge University Press, Amadeus Press and Glitterati Editions, and speaker’s honoraria from Novartis, Biogen, Roche and Sanofi Genzyme. Massimo Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla.
Ethical standards
Approval was received from Institutional ethical standards committees on human experimentation at participating sites (protocol ID: 32/2018). All participants provided written informed consent prior to study participation according to the Declaration of Helsinki.
References
- 1.Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7(12):1139–1151 [DOI] [PubMed] [Google Scholar]
- 2.Mistri D et al (2024) Cognitive phenotypes in multiple sclerosis: mapping the spectrum of impairment. J Neurol 271(4):1571–1583 [DOI] [PubMed] [Google Scholar]
- 3.De Meo E et al (2021) Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol 78(4):414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zackowski KM et al (2021) Prioritizing progressive MS rehabilitation research: a call from the International Progressive MS Alliance. Mult Scler 27(7):989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinstein A et al (2023) Cognitive rehabilitation and aerobic exercise for cognitive impairment in people with progressive multiple sclerosis (CogEx): a randomised, blinded, sham-controlled trial. Lancet Neurol 22(10):912–924 [DOI] [PubMed] [Google Scholar]
- 6.Brandstadter R, Sand IK, Sumowski JF (2019) Beyond rehabilitation: a prevention model of reserve and brain maintenance in multiple sclerosis. Mult Scler J 25(10):1372–1378 [Google Scholar]
- 7.Vollmer TL et al (2021) Multiple sclerosis phenotypes as a continuum the role of neurologic reserve. Neurology-Clinical Practice 11(4):342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocca MA et al (2024) Cognitive rehabilitation effects on grey matter volume and Go-NoGo activity in progressive multiple sclerosis: results from the CogEx trial. J Neurol Neurosurg Psychiatry. 10.1136/jnnp-2024-333460 [DOI] [PubMed] [Google Scholar]
- 9.Bodini B et al (2009) Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum Brain Mapp 30(9):2852–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry RG et al (2008) Regional grey matter atrophy in clinically isolated syndromes at presentation. J Neurol Neurosurg Psychiatry 79(11):1236–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raz E et al (2010) Clinically isolated syndrome suggestive of multiple sclerosis: voxelwise regional investigation of white and gray matter. Radiology 254(1):227–234 [DOI] [PubMed] [Google Scholar]
- 12.Ceccarelli A et al (2008) A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage 42(1):315–322 [DOI] [PubMed] [Google Scholar]
- 13.Filippi M et al (2012) Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures–an explorative study. Radiology 262(3):932–940 [DOI] [PubMed] [Google Scholar]
- 14.Rocca MA et al (2019) Functional and structural plasticity following action observation training in multiple sclerosis. Mult Scler 25(11):1472–1487 [DOI] [PubMed] [Google Scholar]
- 15.Prosperini L, Di Filippo M (2019) Beyond clinical changes: rehabilitation-induced neuroplasticity in MS. Mult Scler 25(10):1348–1362 [DOI] [PubMed] [Google Scholar]
- 16.Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15(4):528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castells-Sanchez A et al (2022) Molecular and brain volume changes following aerobic exercise, cognitive and combined training in physically inactive healthy late-middle-aged adults: the Projecte Moviment randomized controlled trial. Front Hum Neurosci 16:854175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roig-Coll F et al (2024) Changes in cardiovascular health and white matter integrity with aerobic exercise, cognitive and combined training in physically inactive healthy late-middle-aged adults: the “Projecte Moviment” randomized controlled trial. Eur J Appl Physiol 124(3):909–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinstein A et al (2020) Study protocol: improving cognition in people with progressive multiple sclerosis: a multi-arm, randomized, blinded, sham-controlled trial of cognitive rehabilitation and aerobic exercise (COGEx). BMC Neurol 20(1):204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashburner J, Ridgway GR (2012) Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci 6:197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1):95–113 [DOI] [PubMed] [Google Scholar]
- 22.Andersson JLR et al (2017) Towards a comprehensive framework for movement and distortion correction of diffusion MR images: within volume movement. Neuroimage 152:450–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103(3):247–254 [DOI] [PubMed] [Google Scholar]
- 24.Keihaninejad S et al (2013) An unbiased longitudinal analysis framework for tracking white matter changes using diffusion tensor imaging with application to Alzheimer’s disease. Neuroimage 72:153–163 [DOI] [PubMed] [Google Scholar]
- 25.Zhang H et al (2007) High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: an example study using amyotrophic lateral sclerosis. IEEE Trans Med Imaging 26(11):1585–1597 [DOI] [PubMed] [Google Scholar]
- 26.Zhang H et al (2007) Unbiased white matter atlas construction using diffusion tensor images. Med Image Comput Comput Assist Interv 10(Pt 2):211–218 [DOI] [PubMed] [Google Scholar]
- 27.Smith SM et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487–1505 [DOI] [PubMed] [Google Scholar]
- 28.Langdon DW et al (2012) Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS). Mult Scler 18(6):891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocca MA et al (2021) Association of gray matter atrophy patterns with clinical phenotype and progression in multiple sclerosis. Neurology 96(11):e1561–e1573 [DOI] [PubMed] [Google Scholar]
- 30.Driemeyer J et al (2008) Changes in gray matter induced by learning-revisited. PLoS ONE. 10.1371/journal.pone.0002669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mummery CJ et al (2000) A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol 47(1):36–45 [PubMed] [Google Scholar]
- 32.Chan D et al (2001) Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol 49(4):433–442 [PubMed] [Google Scholar]
- 33.Chiaravalloti ND et al (2012) Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol 259(7):1337–1346 [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi K, Sakurai Y (2016) Inactivation of cerebellar cortical crus II disrupts temporal processing of absolute timing but not relative timing in voluntary movements. Front Syst Neurosci. 10.3389/fnsys.2016.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guell X, Gabrieli JDE, Schmahmann JD (2018) Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage 172:437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocca MA et al (2024) Advanced neuroimaging techniques to explore the effects of motor and cognitive rehabilitation in multiple sclerosis. J Neurol 271(7):3806–3848 [DOI] [PubMed] [Google Scholar]
- 37.Tilsley P et al (2023) Physical fitness moderates the association between brain network impairment and both motor function and cognition in progressive multiple sclerosis. J Neurol 270(10):4876–4888 [DOI] [PubMed] [Google Scholar]
- 38.Schneider R et al (2019) Temporal dynamics of diffusion metrics in early multiple sclerosis and clinically isolated syndrome: a 2-year follow-up tract-based spatial statistics study. Front Neurol 10:1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storelli L et al (2022) Advanced diffusion-weighted imaging models better characterize white matter neurodegeneration and clinical outcomes in multiple sclerosis. J Neurol 269(9):4729–4741 [DOI] [PubMed] [Google Scholar]
- 40.Koubiyr I et al (2024) Longitudinal fibre-specific white matter damage predicts cognitive decline in multiple sclerosis. Brain Commun 6(1):fcae018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zivadinov R et al (2018) Effect of dimethyl fumarate on gray and white matter pathology in subjects with relapsing multiple sclerosis: a longitudinal study. Eur J Neurol 25(3):584-e36 [DOI] [PubMed] [Google Scholar]
- 42.Zivadinov R et al (2018) Effect of teriflunomide on gray and white matter brain pathology in multiple sclerosis using volumetric and diffusion-tensor imaging MRI measures. J Neurol Sci 388:175–181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are available one year after publication, upon reasonable request. Please make the request to the corresponding author. A CogEx Committee will review the request for approval. A data sharing agreement will be produced before any data is shared.