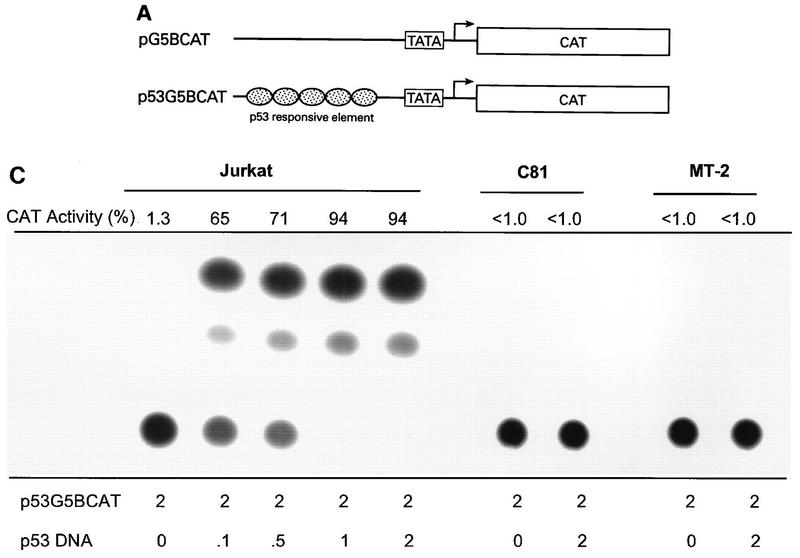
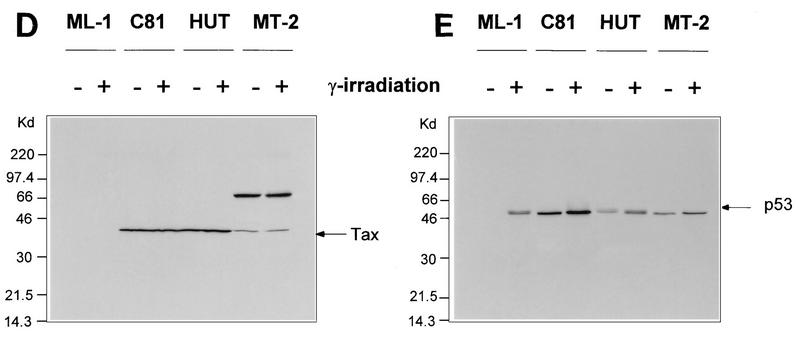
FIG. 1.
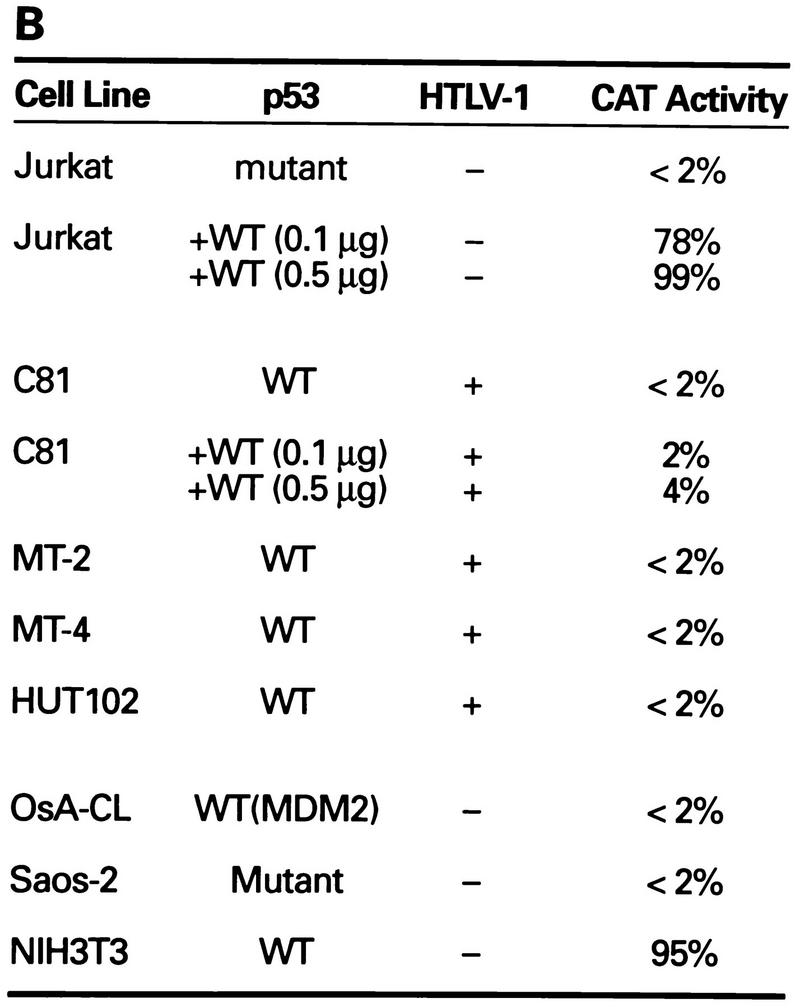
Transcriptional stimulation activity mediated by endogenous p53 protein in HTLV-1 transformed cell lines. (A) Diagrammatic representation of CAT reporter plasmids. The reporter plasmid pG5BCAT contains CAT coding sequences with five Gal4 DNA binding sites upstream of the E1B TATA box. Reporter p53G5BCAT contains six p53 consensus binding sites between the five Gal4 DNA binding sites and the E1B TATA sequence of pG5BCAT. (B) Transcriptional stimulation activity of p53 in different cell lines. pG5CAT or p53G5BCAT (8 μg) was transfected into different cell lines together with 8 μg of GH cDNA (pXGH5). The amounts of protein extract for CAT assays were adjusted to have the same GH activity. In Jurkat and C81 cells, the effect of exogenously introduced wild-type (WT) p53 was assayed by cotransfection of p53G5BCAT DNA (2 μg) with increasing amounts (0.1 and 0.5 μg) of the p53 expression vector. Numbers in the CAT activity column represent percent CAT conversion. (C) p53 transactivation in Jurkat and HTLV-1-transformed cells. p53G5BCAT (2 μg) was transfected into different cell lines together with 8 μg of GH cDNA (pXGH5). The effect of exogenously introduced wild-type p53 was assayed by cotransfection of p53G5BCAT DNA (2 μg) with increasing amounts (0.1 to 2.0 μg) of the p53 expression vector. All transfections were equalized for total DNA by addition of carrier DNA. CAT activity is presented as percent CAT conversion. Level of hGH expression, given as 125I counts per minute obtained in a GH ELISA, were as follows: lane 2, 630; lane 3, 540; lane 4, 423; lane 5, 500; lane 6, 657; lane 7, 558; lane 8, 705; and lane 9, 778. (D and E) Western blot analysis of HTLV-1 Tax and p53 following exposure of normal and HTLV-1-transformed cells to γ irradiation. Cells were lysed in RIPA buffer containing 1 mM PMSF, 1 μg of aprotinin per ml, 1 μg of leupeptin per ml, and 5 mM sodium fluoride at 0°C for 30 min. From each cell lysate, a total of 100 μg of protein was separated on an SDS–10% acrylamide denaturing gel, transferred to an Immobilon membrane (Millipore), and probed with antibodies against Tax (Tab 172) (D) and p53 (PAb421) (E).