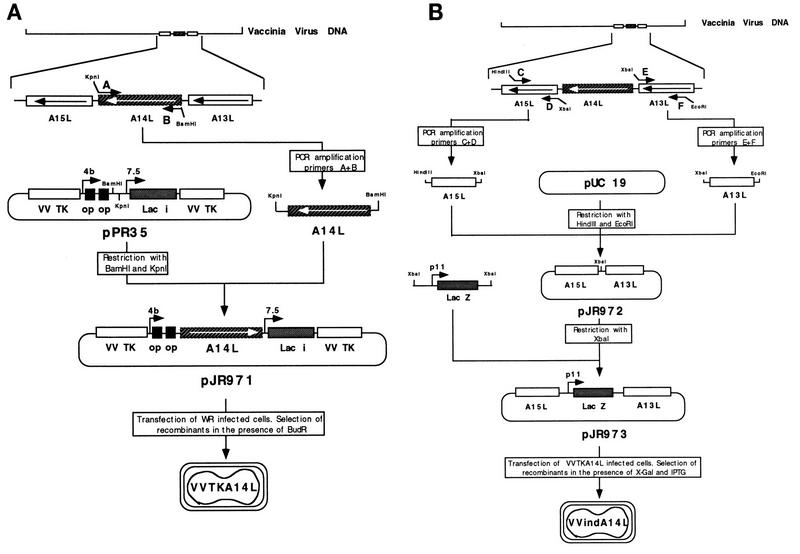
FIG. 1.
Strategy for the construction of the VVindA14L recombinant virus. A two-step strategy was followed for the generation of VVindA14L recombinant virus. (A) Introduction of a lacI operator-regulated copy of the A14L gene in the TK locus of the VV genome. A DNA fragment corresponding to the complete A14L gene was produced by PCR amplification from viral DNA with primers A and B (described in Materials and Methods) and was cloned into the pPR35 plasmid, downstream of a hybrid inducible promoter consisting of the VV p4b promoter fused to two lacI operator (op) units. The resulting plasmid, pJR971, which also contains the lacI repressor gene under the control of the VV p7.5 promoter, was used to transfect BSC40 cells infected with wild-type (WR) virus. TK− intermediate viruses, VVTKA14L, were selected after infection of TK-143 cells in the presence of 5-bromodeoxyuridine (BUdR). (B) Deletion from the VVTKA14L genome of the endogenous A14L gene by replacement with the E. coli lacZ gene. Two DNA fragments corresponding to the left and right A14L flanking sequences were amplified by PCR with VV DNA and primers C-D and E-F, respectively, whose sequences are reported in Materials and Methods, and were both cloned into pUC19 to generate the pJR972 plasmid. A DNA fragment containing the E. coli lacZ gene fused to the VV p11 promoter was introduced into the XbaI site of pJR972, between the two A14L flanking sequences. The resulting plasmid, pJR973, was used to transfect BSC40 cells infected with VVTKA14L virus. Recombinant VVindA14L viruses containing only the inducible A14L gene were selected by the blue plaque phenotype in BSC40 cells infected in the presence of IPTG, after addition of X-Gal.