FIG. 4.
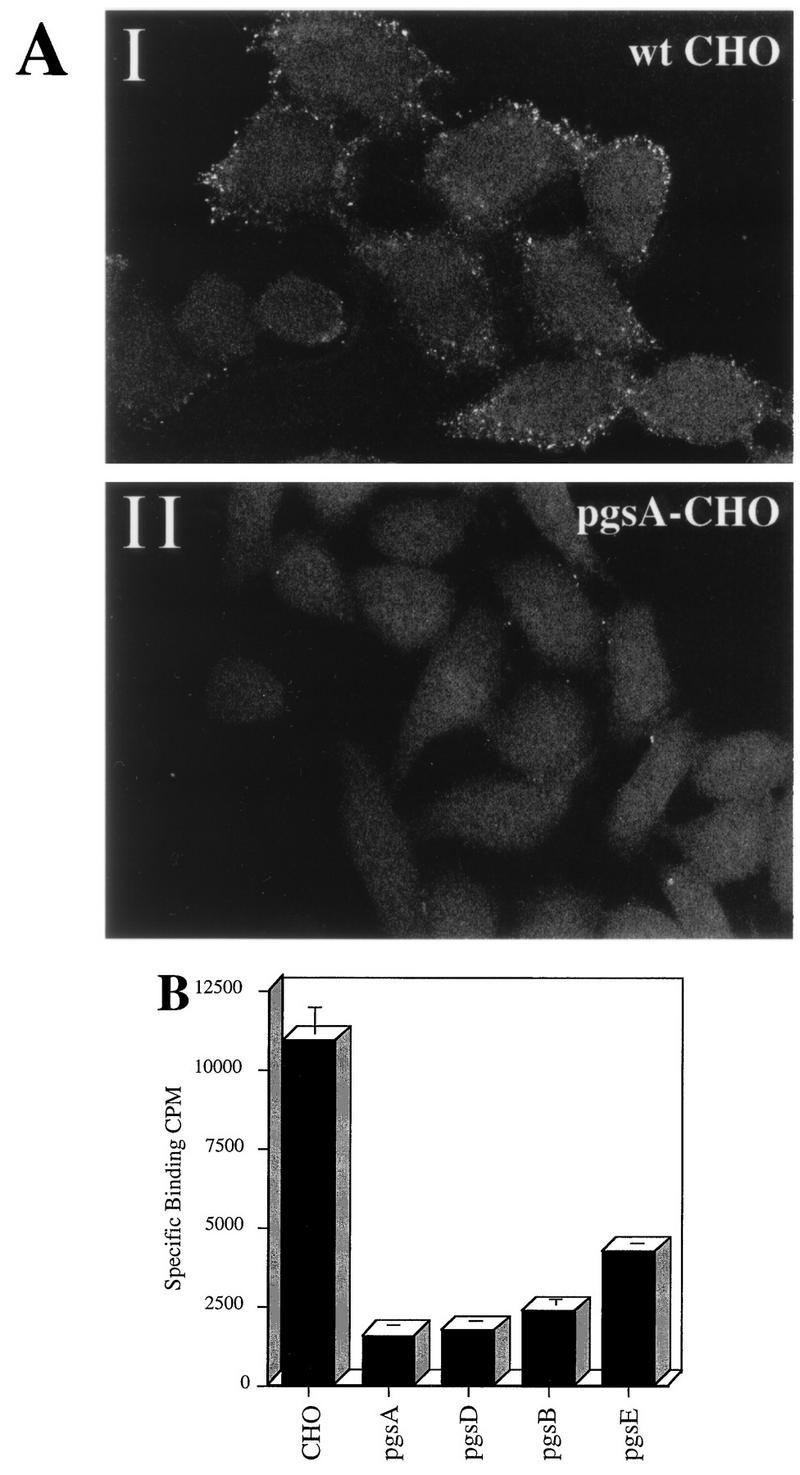
HS proteoglycan serves as a primary attachment receptor for AAV-2. Wild-type CHO-K1 cells and CHO-K1 mutants defective in proteoglycan synthesis were assessed for the ability to bind AAV-2. Cell line pgsA-745 lacks HS and chondroitin sulfate proteoglycans; pgsD-677 lacks HS proteoglycan and produces a threefold excess of chondroitin sulfate proteoglycans; pgsB-618 produces 15% of normal proteoglycans; pgsE-606 produces an undersulfated form of HS proteoglycan and normal levels of chondroitin sulfate proteoglycans. (A) Cy3-labeled AAV-2 was bound to wt CHO cells (I) and the pgsA-745 mutant that lacks proteoglycans (II) as described in Materials and Methods. Images were captured by confocal microscopy. (B) Binding of 3H-AAV to parental and mutant CHO cells. Binding assays were performed at 4°C in Eppendorf tubes. A total of 3 × 105 cells were incubated with 4 × 1011 particles of 3H-AAV for 90 min in HBSB. After thorough washing, cells were pelleted and solubilized, and radioactivity was quantitated as described in Materials and Methods. Nonspecific binding was determined by parallel binding studies done in the presence of a 100-fold excess of unlabeled virus. Data represent the mean specific binding and standard deviation obtained from experiments performed in triplicate.
