Abstract
Introduction
Osteosarcopenic fractures, an emerging geriatric syndrome characterized by sarcopenia-osteoporotic fractures coexistence, delayed fracture healing, and elevated risk of re-fracture. Limited research has investigated the mechanisms by which skeletal muscle satellite cells (SMSCs) promote muscle regeneration and osteoporotic fracture healing. The aim of this study was to investigate the impact of a traditional herbal decoction (HD), the Invigorate the Spleen and Tonify the Kidney Formula, on SMSC regulation, muscle regeneration, and fracture healing.
Method
Using conditional knockout mice, the role of SMSCs in promoting fracture healing and mitigating sarcopenia was evaluated by visualizing the fracture area and surrounding muscle tissue. The signaling pathways involved were comprehensively analyzed using a combination of Western blotting, real-time PCR analysis, immunohistochemical staining, and immunofluorescent staining. And the key elements and compounds facilitating osteogenesis and myogenesis were identified using HPLC and network pharmacology analysis.
Results
This study demonstrated that the herbal decoction mediates the β-catenin signaling pathway, mobilizes SMSCs to migrate to the fracture area, facilitates their osteogenic and myogenic differentiation, and enhances osteoporotic fracture healing. Knockdown of β-catenin in SMSCs in Pax7-CreERT2/+;β-cateninfx/fx conditional knockout mice led to sarcopenia and osteoporosis. Additionally, the herbal decoction significantly increased bone mass, repaired bone microstructure, and promoted muscle fiber remodeling around fractures in mice.
Conclusions
These findings provide the first evidence that the HD, as a β-catenin agonist, not only promotes fracture healing by modulating the osteogenic and myogenic effects of SMSCs but also ameliorates sarcopenia. This study offers practical evidence supporting the formula as a promising therapeutic candidate for treating osteosarcopenic fractures.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-025-04642-6.
Keywords: Osteosarcopenia, Osteoporotic fracture, Skeletal muscle stem cells, Herbal decoction, β-catenin
Introduction
Osteosarcopenic fractures, an emerging geriatric syndrome characterized by sarcopenia-osteoporotic fractures coexistence. Sarcopenia is a common condition characterized by the progressive loss of skeletal muscle mass and function, leading to reduced physical mobility, diminished quality of life, and an increased risk of negative effects like fractures, falls, and premature death [1]. Fractures are also a severe complication of osteoporosis. Osteoporotic fractures (OPFs), also known as fragility fractures, are among the most prevalent global conditions [2]. The balance between bone formation and bone resorption, which is crucial for maintaining skeletal integrity, is tightly regulated by osteoclasts, osteoblasts, and osteocytes [3]. A breakdown of this balance can lead to pathologically altered bone quality and structure [4, 5]. No pharmacological agents currently address osteosarcopenic fractures.
In 2017, an estimated 2.68 million new fragility fractures occurred in six European countries (United Kingdom, Italy, France, Germany, Sweden, and Spain), highlighting that more than 0.3 billion individuals aged ≥ 50 years worldwide may face an increased risk of osteoporotic fractures by 2040 [6, 7]. While surgical intervention is the primary therapeutic approach for treating OPFs, reducing mortality and improving physical function in many patients [8]. While the importance of muscle-derived signals in bone formation is increasingly recognized, the specific cellular and molecular mechanisms mediating this crosstalk, remain incompletely understood [9]. Given the substantial social and economic burden associated with OPFs, improving treatment strategies for high-risk populations is essential. Specifically, regulating mesenchymal stem cell (MSC) differentiation to promote bone regeneration is critical for improving therapeutic outcomes in bone diseases.
In contrast to the extensively studied bone marrow mesenchymal stem cells, the effect of skeletal muscle satellite cells (SMSCs) in facilitating fracture healing remains underexplored. SMSCs are situated in the muscle fibres between the plasma membrane and the basement membrane [10]. While typically quiescent, SMSCs exhibit mesenchymal stem cell activity following stimulation [11, 12]. Paired box 7 (Pax7) is a critical molecular marker for SMSC formation during embryogenesis [13]. Additionally, 5-bromodeoxyuridine (BrdU), a thymidine analog, is widely used in studies related to cell proliferation [14]. Although SMSCs have been reported to differentiate into osteoblasts, research on this topic remains limited [15, 16]. Notably, prior findings have shown that SMSCs promote osteoporotic fracture healing via β-catenin, suggesting its potential as a therapeutic target [17].
β-catenin signaling is often dysregulated in individuals with muscle deficiencies (e.g., myopathy and atrophy) due to genetic or epigenetic factors [18, 19]. β-catenin interacts with myogenic differentiation (MyoD) and activates target genes [20]. Additionally, WNT5a, a crucial WNT ligand, can inhibit adipogenic differentiation of progenitor cells through the positive regulation of β-catenin signaling, enhancing their pro-myogenic effects [21]. β-catenin is also a critical regulator of skeletal homeostasis and development, with its dysregulation reported during aging [22, 23].
For this study, a traditional Chinese herbal decoction (HD), the Invigorate the Spleen and Tonify the Kidney Formula, was selected as a β-catenin agonist. HD has demonstrated potential in modulating SMSCs and promoting fracture healing while offering convenience and biological compatibility. However, its mechanism remains unclear.
This study utilized Pax7-CreERT2/+;β-cateninfx/fx conditional knockout(cKO) mice to specifically knock out β-catenin in SMSCs. In vivo experiments were conducted to investigate the correlation between muscle and bone and to observe the effects of HD on the bone and muscle of cKO mice. The objective of this work was to elucidate the mechanism by which HD-mediated β-catenin signaling regulates SMSCs to promote myogenic and osteogenic differentiation for treating osteosarcopenic fractures.
Material and methods
Mouse experiments and ethical statement
Thirty-two 1-month-old C57BL/6 mice were obtained from the Shanghai Model Organisms Center, Inc. They were screened based on their specific pathogen-free (SPF) grade and maintained under standard conditions with freely available water and food. The mean weight of the animals was recorded as 31.9 ± 4.5 g.
Crossing the C57BL/6 J-background Pax7 CreERT2/+ mice with β-cateninfx/fx mice resulted in the generation of Pax7 CreERT2/+;β-cateninfx/fx mice. Genotyping was performed using agarose gel electrophoresis of genomic DNA extracted from the tails of 3-week-old mice (Supplemental Fig. 1A). Body weights and general health signs were monitored for all groups before fracture modeling, with no significant differences observed (Supplemental Fig. 1B and 1C).
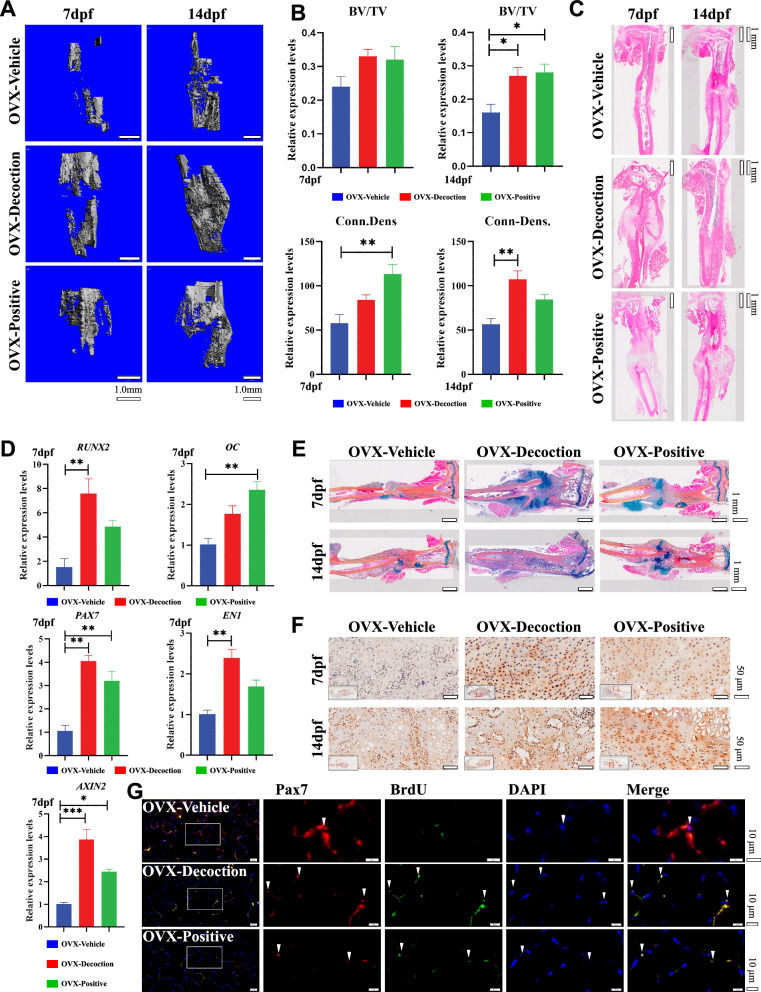
Fig. 1.
HD mediates β-catenin to promote fracture healing. (A) Micro-CT 3D reconstruction at 7 dpf and 14 dpf. Bar, 1 mm. (B) Micro-CT quantitative analysis at 7 dpf and 14 dpf. Results are shown as mean ± SEM (n = 5). *P < 0.05, **P < 0.01. (C) The tibial fracture site by H&E staining, was treated for 7 dpf and 14 dpf. Bar, 500 μm. (D) RT-PCR results of tibialis posterior muscle-related genes in the OVX compound fracture model mice. Results are shown as mean ± SEM (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001. (E) The tibial fracture site by Alcian Blue/hematoxylin and orange G staining after treatment for 7 dpf and 14 dpf. Bar, 500 μm. (F) Immunohistochemical β-catenin staining: At 7 dpf and 14 dpf, the fracture sites of each group were stained. (G) Immunofluorescence staining (Pax7 + Brdu) of the posterior tibial muscle showed that the positive expression of SMSCs in the OVX-Decoction and OVX-Positive group was significantly higher than that of OVX-Vehicle group
Animal experiments were conducted following the guidelines of the "Guide for the Care and Use of Laboratory Animals" issued by the National Institutes of Health (NIH). The experimental protocols were approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine (Ethics Approval No: PZSHUTCM190329005).
Experimental groups
Individual identification of the 30 C57BL/6 female mice was achieved via toe clipping and subsequent numbering from 1 to 30. Randomization was performed using SPSS 24.0 software, creating three distinct groups: Vehicle, Decoction, and Positive. Every group consists of two points in time (7 and 14 days post-fracture [dpf], n = 5).
Additionally, a total of 20 litter-negative β-cateninfx/fx mice comprised the Con-Vehicle group and 60 Pax7 CreERT2/+;β-cateninfx/fx mice were randomly divided into TM-Vehicle, TM-Decoction, and TM-Positive. The assessment takes place at two points in time (7 dpf and 14 dpf) for each group(n = 10).
Model establishment and evaluation of osteoporosis
To activate Pax7 CreERT2/ + in the TM group, 1-month-old mice received intraperitoneal injections of tamoxifen (100 mg/kg dissolved in corn oil) once daily for five consecutive days. Preliminary micro-computed tomography (micro-CT) showed that knockdown of β-catenin in SMSCs resulted in a significantly reduction in bone mineral density (BMD) in the TM group (P < 0.001; Supplemental Figs. 1D and 1E). Bone microstructure at the tibial plateau was also significantly impaired(Supplemental Figs. 1F).
Construction of an OPF model
Thirty 3-month-old female C57BL/6 mice underwent ovariectomy (OVX) to induce osteoporosis, followed by experimental procedures three months post-surgery. Tibial fracture modeling was performed under isoflurane anesthesia in a sterile environment. The procedure involved hair removal and skin sterilization with iodine [24]. Muscle and fascia were bluntly stripped, and a Kirschner wire was inserted into the proximal tibia. Transverse osteotomy was then performed, and the tibia was stabilized using the Kirschner wire. Finally, the incision was closed in layers (Supplemental Fig. 1E).
Drug formulation
The herbal decoction was prepared and identified by pharmacists at Longhua Hospital following standard procedures (Table 1). According to the Chinese Medical Pharmacopoeia, all herbs were steeped in 12 L of water for 30 min, boiled for 40 min, and then filtered. The residue was reboiled in 8 L of water for another 40 min and filtered again. The filtrates were combined and prepared for oral administration. Pre-experimentally, OVX surgery was performed on 3-month-old female mice, and postoperatively, the treatment group was subjected to a compound intervention experiment with different doses (high, medium and low), and we found that the medium-dose group of the herbal decoction was the optimal group (Supplemental Figs. 1G).
Table 1.
Composition of herbal decoction
| Latin name | Chinese name | Amount (g) | Lot No | Place of origin | Company |
|---|---|---|---|---|---|
| Codonopsis Pilosula | Dangshen | 12 | 190,822–1 | Shanxi, China | Shanghai WanShiCheng Chinese Medicine Co. Ltd |
| Drynaria Fortunei | Gusuibu | 9 | 2,019,062,802 | Chongqing, China | Shanghai DeHua Chinese Medicine Co. Ltd |
| Acanthopanax Senticosus | Ciwujia | 12 | 20,200,811 | Changbai, China | Linjiang Tranquil Local Product Shop |
| Epimedium Brevicornum | Yinyanghuo | 9 | 2,019,082,006 | Gansu, China | Shanghai DeHua Chinese Medicine Co. Ltd |
| Salvia Miltiorrhiza | Danshen | 12 | 200,706 | Shandong, China | Shanghai Hongqiao traditional Chinese medicine decoction pieces Co., Ltd |
| Angelica Pubescens F. Biserrata | Duhuo | 9 | 20,200,515–1 | Hubei, China | Shanghai WanShiCheng Chinese Medicine Co. Ltd |
The voucher specimens were deposited at Longhua Hospital, affiliated with Shanghai University of TCM
Dosing was determined using the "Methods of Pharmacology of New Chinese Medicines," with the conversion formula for mice as follows: the dose of mice = 1/70*10*weight*number*day. Mice received 0.2 mL/day by gavage for 7 or 14 days, beginning the second day after tibial fracture modeling. In the Decoction group, mice were administered the herbal decoction. The Positive group received Strong Bone Capsules, an anti-osteoporosis drug containing total flavonoids (National Drug Standard: WS3-891(Z-176)-2004(Z)). The Vehicle group received 0.9% saline. Free access to water for all groups.
X-ray and micro-CT
The left tibia was carefully dissected, with adjacent muscles removed, and immersed in buffered formalin before being replaced with 75% ethanol for micro-CT scanning. X-ray images of the left tibia were captured at 55 kVp and 71 μA. Quantitative bone histomorphometric analyses included trabecular trabecular number (Tb.N), thickness (Tb.Th), connectivity density (Conn.Dens), and bone volume/total volume (BV/TV).
Histopathological examination
Muscle tissue was fixed in a fixative for 24 h, dehydrated, embedded in paraffin, and sectioned sagittally and horizontally (4 μm thick). Decalcified femoral sections were stained with hematoxylin and eosin (H&E) and Masson's stain. After confirming decalcification by X-ray, tibial sections were dehydrated, embedded in paraffin, and subjected to continuous sagittal sectioning (4 μm thick). These sections were stained with H&E, Alcian blue/hematoxylin and orange G (ABHO), Safranin-fast green, and subjected to immunofluorescence and immunohistochemistry. Target areas were analyzed using Olympus VS120-SL image analysis software after scanning the entire film.
Real-time PCR analysis
Muscle tissue was ground into a powder in its frozen state. β-actin is the reference gene. The following primer sets (all sequences 5′–3′) were used: Runx2-F, AACGATCTGAGATTTGTGGGC; Runx2-R, CCTGCGTGGGATTTCTTGGTT; Osteocalcin(OC)-F, GACCGCCTACAAACGCATCTA; OC-R:GACAGGGAGGATCAAGTCCCG; Pax7-F, CGATTAGCCGAGTGCTCAGA; Pax7-R:GGAGGTCGGGTTCTGATTCC; Engrailed 1(EN1)-F, CCGGTGGTCAAGACTGACTC; EN1-R, CCGCTTGTCTTCCTTCTCGT; Axin2-F, ATAAGCAGCCGTTCGCGATG; and Axin2-R, CTCTCTTAAGTCAGCAGGGGC. Relative quantity by the 2−ΔΔCt method.
Immunohistochemical staining
Deparaffinized slices were soaked in methanol:H₂O₂ (1:9) for 15 min, followed by collagen extraction using collagenase from Clostridium histolyticum. Sections were incubated in a 37 °C thermostat for 15 min. It was blocked with 5% bovine serum albumin (BSA) for 20 min and then incorporated rabbit anti-β-catenin and goat anti-rabbit IgG antibodies. The avidin/biotin enzyme complex peroxidase was added for 15 min, and the sections were visualized using a 3,3′-diaminobenzidine color development solution under an Olympus VS120-SL microscope at 20 × magnification.
Immunofluorescent staining
Dewaxed sections underwent antigen retrieval using collagenase from Clostridium histolyticum. Slides were stagnated with 5% BSA for 20 min and cultured overnight with rabbit primary antibodies: (Pax7: 1% BSA 1:200), BrdU (BrdU: 1% BSA 1:200), MyoD (MyoD: 1% BSA 1:200) and Sca1 (Sca1: 1% BSA 1:200). Anti-rabbit IgG (488: 5% BSA 1:500 + 555: 5% BSA 1:500) was added, and the sections were stained for 1 h (in the dark). Visualized under an Olympus VS120-SL microscope.
Muscle wet weight coefficient
Prior to tissue harvesting, mice were weighed. Harvesting procedure: The muscles of the operated hindlimb, including the gastrocnemius, soleus, and tibialis posterior muscles, were dissected. The wet weight of each individual muscle was recorded. Subsequently, the ratio of each muscle's wet weight to the terminal body weight was calculated.
Western blot
Posterior tibial muscles were extracted post-treatment, weighed, and preserved at − 80 °C. Proteins were extracted using the BCA method and stored at − 20 °C. Equivalent protein amounts were isolated by 8–12% SDS-PAGE and moved to PVDF. After blocking with 5% BSA in PBST, primary antibodies (β-catenin 1:4000, MyoD1 1:4000, MEF2C 1:4000, GAPDH 1:4000) were incubated overnight in 1% BSA. Membranes were then incubated with secondary antibodies (1% BSA, 1:1000/1:10,000) for 2 h.
HPLC-Q-TOF of herbal decoction
HD components were characterized using an Agilent 1290 UPLC system coupled with an Agilent Q-TOF 6545 LC/MS. The mobile phase consisted of solvent A (acetonitrile) and solvent B (water with 0.1% formic acid) at a flow rate of 0.3 mL/min. Separation was performed on a Waters CORTECS® UPLC® T3 column (2.1 × 100 mm, 1.6 μm) at 30 °C. Data were analyzed using MassHunter Workstation Qualitative Analysis Software (version B.07.00).
Network pharmacology
Active ingredients of herbal components (e.g., Codonopsis pilosula, Drynaria fortunei, Epimedium brevicornum, and Salvia miltiorrhiza) were identified using the TCMSP database (https://tcmsp-e.com/) with oral bioavailability ≥ 30% and drug-likeness (DL) ≥ 0.18. Candidate genes were obtained using the BATMAN-TCM database (http://bionet.ncpsb.org.cn/batman-tcm/) with score cutoff > 0.99 and P < 0.05. Duplicate targets were removed, and standardized gene names were verified with the UniProt database (https://www.uniprot.org/). Disease-related targets were identified by searching for "osteoporotic fracture" in GeneCards (https://www.genecards.org/) and OMIM (https://www.omim.org/). Drug-component-target networks were constructed using Cytoscape 3.9.1 software.
GO enrichment analysis and KEGG pathway analysis
Drug-disease crossover genes were analyzed in the DAVID database (https://david.ncifcrf.gov/) for Gene Ontology (GO) terms, including biological process (BP), cellular component (CC), and molecular function (MF). The top 10 BP, CC, and MF terms, and the top 20 KEGG pathways related to osteoporotic fractures, were selected (P < 0.01).
Other agents
Strong bone capsule (cat. #Z20030007) was purchased from Beijing Qihuang Pharmaceutical Co., Ltd. (Beijing, China); RIPA Lysis Solution (cat. #P0013B), SDS-PAGE protein loading buffer(cat. #P0015L), BeyoECL star (cat. #P0018AS), SDS-PAGE electrophoresis solution(cat. #P0014D), SDS-PAGE gel kit (cat. #P0012AC) and Western Transfer Film (cat. #P0021B) were from Beyotime (Shanghai China); MASSON kit (cat. #G1006) and GD fixative (cat. #G1111) were from Servicebio (Wuhan, China); Anti GAPDH antibody (cat. #ab181602), Anti β-catenin antibody(cat. #ab6302), Goat anti rabbit (cat. #ab205718), Anti BrdU antibody (cat. #ab6326), Anti Sca1 antibody (cat. #ab25031) and Anti-Pax7 antibody (cat. #ab187339) were from Abcam (UK); Anti MYOD antibody was purchased from Novus (UK) (cat. #NBP1-54,153); Tamoxifen (cat. #T5648), Clostridium histolyticum (cat. #C6885), Eosin (cat. #E4009-5G), Hematoxylin (cat. #H-3136), Alcian Blue (cat.#A5268), Orange G (cat. #1936–15-8), Phloxine B (cat. #18,472–87-2), Dimethyl sulfoxide (DMSO) (sigma amresco cat.#67–68-5), Albumin BovineV (sigma Roche cat.#10,735,078,001) and Trypsin (cat. #T7409) were from Sigma (USA); ABC-HRP Kit (cat. #PK-6100), Antifade Mounting Medium with DAPI (cat. #H-1200) and DAB Substrate Kit (cat. #SK-4100) were from Vectorlabs (USA); Anti-rabbit IgG (H + L) 555 (cat. #4413/4409) and Anti-rat IgG (H + L) 488 (cat. #4413) were from Cell Signaling Technology (CST) (USA).
Statistical analysis
Data are expressed as mean ± standard error. Assuming a 30% biologically significant difference with 10–20% variability, α and 1 − β values were set at 0.05 and 0.8, respectively. No animals or samples were excluded from the analysis. For group comparisons, one-way ANOVA with Least Significant Difference (LSD) post-hoc tests was used for data passing normality tests; otherwise, the Kruskal–Wallis test followed by Dunn's Multiple Comparison Test was applied. Analyses were performed using SPSS 24.0, with P < 0.05 considered significant (*P < 0.05, **P < 0.01, ***P < 0.001). Graphical representations were created using GraphPad Prism 8.
Results
HD mediates β-catenin to promote OPF healing
Following pharmaceutical intervention in a conventional OVX mouse model of compound fractures, micro-CT scanning visualized bone callus structure and enabled three-dimensional reconstruction mapping. Comparative analysis showed that, relative to the OVX-Vehicle group, all drug-treated groups exhibited enhanced bone callus generation and maturation at 7 and 14 days post-fracture (dpf). Notably, the HD group demonstrated the most pronounced effect (*P = 0.0226, **P < 0.01, Fig. 1A and 1B). Histological examination using HE staining revealed that at 7 dpf, OVX-Decoction and OVX-Positive groups promoted cartilaginous callus formation at fracture sites, whereas minimal scab formation was observed in the saline control group. By 14 dpf, enhanced callus remodeling was evident in the drug-treated groups, while callus formation had only commenced in the OVX-Vehicle group (Fig. 1C).
RT-PCR analysis of tibialis posterior muscle showed that the OVX-Decoction group significantly upregulated the expression of Runx2, a bone formation-related signaling gene (**P < 0.01, Fig. 1D). Additionally, enhanced expression levels of Pax7, a marker gene for myogenic stem cells, and Engrailed 1 (EN1), a transcription factor regulating cell development, were observed. The treatment also promoted the expression of Axin2, a key downstream target of β-catenin (**P < 0.01, ***P < 0.001, Fig. 1D). ABHO staining at the fracture site indicated that all groups facilitated chondrocyte (cartilaginous callus) formation at fracture ends within 7 dpf. By 14 dpf, callus remodeling was evident in the OVX-Decoction and OVX-Positive groups, accompanied by a substantial presence of apoptotic chondrocytes and increased osteoblast populations (Fig. 1E). Immunostaining for β-catenin revealed pronounced cytoplasmic and nuclear β-catenin expression in chondrocytes within the bone callus across all treatment groups (Fig. 1F). Immunofluorescence double staining for Pax7 and BrdU in the gastrocnemius muscle showed that early HD intervention enhanced Pax7 expression in skeletal muscle, indicating increased SMSC proliferation and enhanced repair of bone and muscle injuries (Fig. 1G). These results suggest that HD promotes healing of fractures by facilitating the β-catenin and regulating chondrocyte and osteoblast proliferation and differentiation.
Knockdown of β-catenin in SMSCs with sarcopenia
In terms of the muscle wet weight coefficient, the TM-Vehicle group displayed significantly lower values compared to the Con-Vehicle group at 7 dpf (*P = 0.0248, ***P < 0.001). By 14 dpf, the TM-Decoction and TM-Positive groups showed increased muscle wet weight ratios relative to the TM-Vehicle group, with the TM-Decoction group demonstrating significantly greater effectiveness (*P = 0.0405). HD treatment improved muscle wet weight in β-catenin knockout conditions but did not surpass levels in the Con-Vehicle group, suggesting that β-catenin is a key signaling pathway in HD-mediated effects (Fig. 2A and 2B). H&E staining results at 7 dpf revealed larger muscle interspaces in the TM-Vehicle, TM-Decoction, and TM-Positive groups compared to the Con-Vehicle group in both cross-sectional and sagittal views. Conversely, muscle fibers were more compact in the Con-Vehicle group than in the TM groups. By 14 dpf, muscle relaxation improved in the TM-Decoction and TM-Positive groups, though not to the level observed in the Con-Vehicle group (Fig. 2C and 2D).
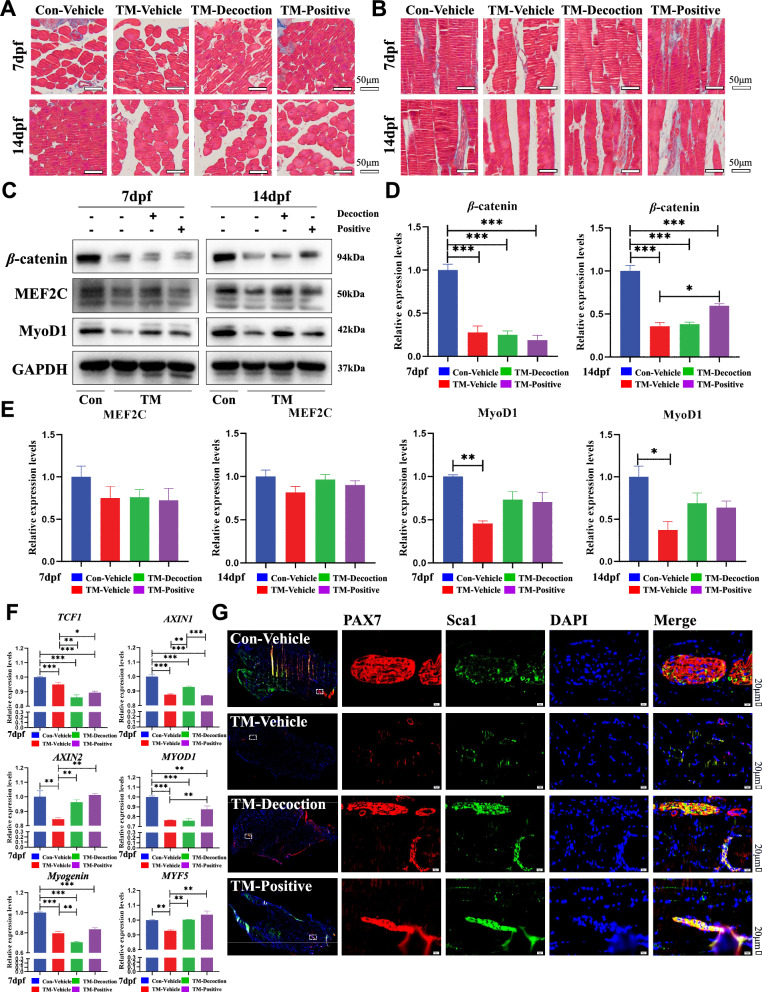
Fig. 2.
Knockdown of β-catenin in SMSCs with sarcopenia. (A) The muscle coefficient ratio of the four groups. (B) Statistical results of muscle coefficient ratio of four groups. Results are shown as mean ± SEM (n = 10). *P < 0.05, ***P < 0.001. (C) H&E staining of the left soleus muscles (cross-section). Bar, 50 μm. (D) H&E staining of the left soleus muscle (longitudinal section). Bar, 50 μm. (E) β-catenin immunohistochemical staining of the left soleus muscle of the four groups. The retest of the knockout efficiency of the TM group. Bar, 50 μm. (F) Related quantitative analysis of β-catenin immunohistochemical staining at 7dpf. Results are shown as mean ± SEM (n = 10). ***P < 0.001. (G) Related quantitative analysis of β-catenin immunohistochemical staining at 14dpf. Results are shown as mean ± SEM (n = 10). ***P < 0.001. (H) Immunofluorescence staining (MyoD + BrdU) of the posterior tibial muscle on the model side at 7 dpf. Bar, 20 μm
Immunohistochemical staining for β-catenin in muscle showed lower protein expression in the TM groups compared to the Con-Vehicle group. HD treatment upregulated β-catenin expression at 14 dpf, but levels remained significantly lower in the TM group relative to the Con-Vehicle group, confirming the knockdown of β-catenin (Fig. 2E–2G). Immunofluorescence staining revealed that HD treatment promoted the expression of MyoD and BrdU, indicating enhanced muscle cell proliferation and repair capacity, although the effect was comparable to that of the Con-Vehicle group (Fig. 2H and Supplemental Fig. 2A). These findings suggest that β-catenin knockdown in SMSCs may lead to sarcopenia.
Up-regulation of β-catenin regulates SMSCs and promotes myogenic differentiation
Masson's staining in horizontal and sagittal planes revealed that at 7 dpf, collagen fiber deposition was markedly increased in the Con-Vehicle and TM-Positive groups compared to the TM-Vehicle and TM-Decoction groups. At 14 dpf, no significant differences were observed in horizontal plane staining results, while sagittal plane results displayed significantly more intense collagen staining in the Con-Vehicle, TM-Decoction, and TM-Positive groups. These findings suggested that After knocking out β-catenin, HD did not significantly promote the formation of collagen fibres in the muscles near the fracture site (Fig. 3A and 3B). In the tibialis posterior muscle, β-catenin expression was significantly reduced in the three TM groups compared to the Con-Vehicle group (***P < 0.001), indicating efficient gene knock down. At 14 dpf, β-catenin expression increased in both TM-Decoction and TM-Positive groups, though the TM-Decoction group exhibited less pronounced effects than the TM-Positive group (Fig. 3C and 3D). Detection of myogenic differentiation markers, MyoD1 and MEF2C, demonstrated improvements in both TM-Decoction and TM-Positive groups at 14 dpf, with the TM-Decoction group showing the most significant effects (Fig. 3E).
Fig. 3.
Up-regulation of β-catenin regulates SMSCs and promotes myogenic differentiation. (A) Masson’s staining of left soleus muscles (cross-section). Bar, 50 μm. (B) Masson’s staining of left soleus muscles (longitudinal section). Bar, 50 μm. (C) Western blot analysis of muscle β-catenin knockout efficiency and detection of myogenic differentiation indicators MEF2C and MyoD1. (D) Muscle β-catenin analysis of Western blot. Results are shown as mean ± SEM (n = 6). ***P < 0.001. (E) Myogenic differentiation analysis of Western blot. Results are shown as mean ± SEM (n = 6). *P < 0.05, **P < 0.01. (F) RT-PCR results of tibialis posterior muscle-related genes in the Pax7 CreERT2/+;β-cateninfx/fx compound fracture model mice. Results are shown as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001. (G) Immunofluorescence staining (Pax7 + Sca1) of the posterior tibial muscle on the model side at 7 dpf. Bar, 20 μm
Muscle RT-PCR analysis revealed statistically significant reductions in β-catenin downstream targets TCF1, AXIN1, and AXIN2 in the TM group compared to the Con-Vehicle group (***P < 0.001). Additionally, the expression of myogenic-related genes MYOD1, Myogenin, and MYF5 was reduced in the TM group and showed no significant increase in either the TM-Decoction or TM-Positive groups (**P < 0.01, ***P < 0.001, Fig. 3F). Pax7, a critical molecular marker of SMSCs, exhibited remarkable expression increases in TM-Decoction, as observed in Pax7 + Sca1 and Pax7 + BrdU staining (Fig. 3G and Supplemental Fig. 2B-2D). These findings indicated that β-catenin played a crucial role in the complex modulation of SMSC proliferation and differentiation by HD.
Knockdown of β-catenin in SMSCs results in osteoporosis and delayed bone healing
At 7 dpf, X-rays revealed a clear fracture line in all groups. At 14 dpf, the TM-Vehicle group showed no significant callus connection at the fracture site, while the fracture line in the Con-Vehicle group began to blur, and abundant callus formation was observed at the fracture ends. Both the TM-Decoction and TM-Positive groups exhibited partial improvements (Fig. 4A). Micro-CT analysis at 7 dpf showed no marked differences in 3D reconstruction images among the groups (Fig. 4B). However, quantitative analysis revealed significantly lower BV/TV values in the TM-Vehicle group compared to the Con-Vehicle group (Fig. 4C). At 14 dpf, 3D reconstruction images displayed obvious callus formation in the Con-Vehicle group, with minor improvements in the TM-Decoction and TM-Positive groups, though neither exceeded the Con-Vehicle group. Quantitative analyses demonstrated significantly lower BV/TV, Conn-Dens, and Tb.Th values in the TM-Vehicle versus the Con-Vehicle group (***P < 0.001). Differences between the TM-Decoction and TM-Positive groups and the TM-Vehicle group were statistically significant (**P < 0.01; *P < 0.05), yet remained below the Con-Vehicle group (Fig. 4D).
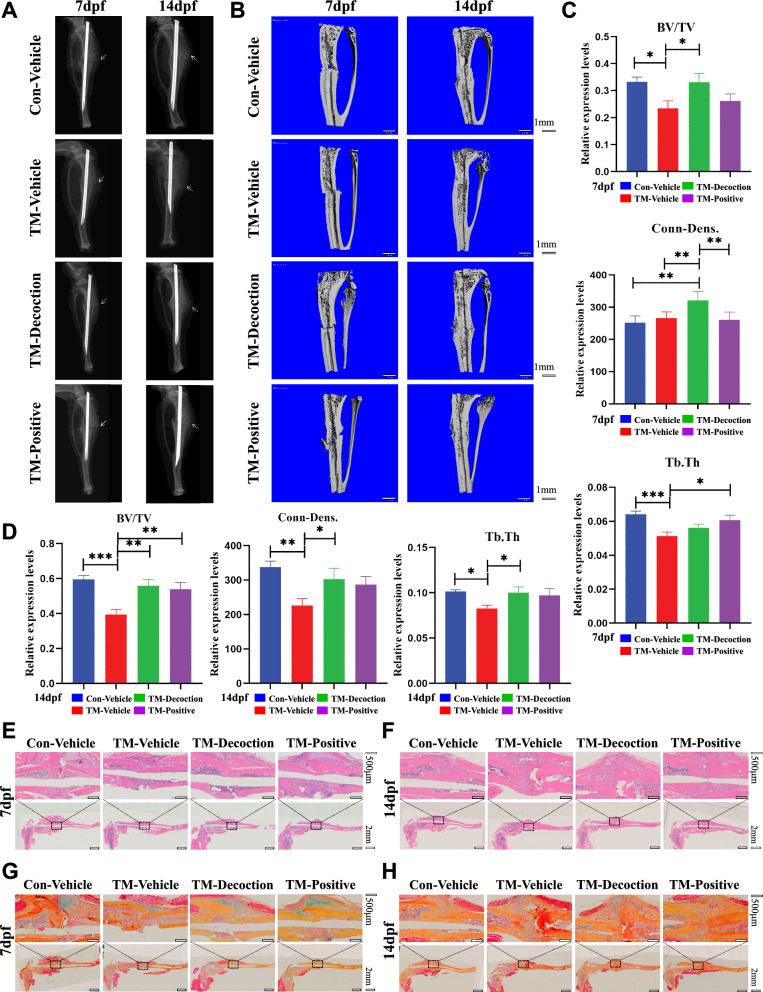
Fig. 4.
Knockdown of β-catenin in SMSCs results in osteoporosis and delayed bone healing. (A) X-ray scan of the left tibia at 7 dpf and 14 dpf. (B) Micro-CT 3D reconstruction at 7 dpf and 14 dpf. Bar, 1 mm. (C) Micro-CT quantitative analysis at 7 dpf. Results are shown as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001. (D) Micro-CT quantitative analysis at 14 dpf. Results are shown as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001. Analysis of tibial fracture site by H&E staining, treated for 7 dpf (E) and 14 dpf (F). Bar, 500 μm. Analysis of tibial fracture site by Alcian Blue/hematoxylin and orange G staining after treatment for 7 dpf (G) and 14 dpf (H). Bar, 500 μm
H&E staining at 7 dpf showed callus formation at fracture ends in the Con-Vehicle, TM-Decoction, and TM-Positive groups, but no callus formation in the TM-Vehicle group (Fig. 4E). At 14 dpf, cartilage callus increased in the TM-Decoction and TM-Positive groups, while the Con-Vehicle group displayed substantial callus formation(Fig. 4F). ABHO staining at 7 dpf revealed relatively less chondrocytes formation in the TM-Vehicle group, while the Con-Vehicle, TM-Decoction, and TM-Positive groups exhibited chondrocytes proliferation at fracture sites (Fig. 4G). By 14 dpf, residual chondrocytes persisted in the Con-Vehicle, TM-Vehicle, and TM-Decoction groups, whereas chondrocytes were largely absent in the TM-Positive group, accompanied by substantial hard callus formation (Fig. 4H). H&E and ABHO pathological staining demonstrated that β-catenin knockdown in SMSCs caused significant delays in callus formation, cartilage development, and osteogenesis. These findings suggested that β-catenin knockdown in SMSCs could result in osteoporosis and delayed bone healing. Concomitantly, unlike observations in OVX mice, HD failed to significantly potentiate osteoporotic fracture healing upon β-catenin knockdown in SMSCs. This inverse evidence indicates that HD likely mediates its pro-healing effects in osteoporosis through β-catenin-dependent mechanisms.
Up-regulation of β-catenin controls the osteogenic differentiation of SMSCs and enhances the recovery of OPF
Safranin-fixed Green staining revealed a significant increase in cartilage formation in the TM-Decoction and TM-Positive groups at 14 dpf. Meanwhile, cartilage was also observed in the Con-Vehicle group, but no significant cartilage was formed in the TM-Vehicle group (Fig. 5A). Immunohistochemical staining for osteoprotegerin (OPG) demonstrated visible callus areas and remarkable OPG-positive staining in the cytoplasm of the Con-Vehicle group at 7 dpf, significantly differing from the TM-Vehicle, TM-Decoction, and TM-Positive groups (***P < 0.001; Fig. 5B and 5C). By 14 dpf, the TM-Decoction and TM-Positive groups exhibited notably higher OPG levels comparing with the TM-Vehicle group (***P < 0.001; Fig. 5C).
Fig. 5.
Up-regulation of β-catenin regulates osteogenic differentiation of SMSCs and promotes healing of OPF. (A) Analysis of tibial fracture site by Safranin-fixed green staining, treated for 7 dpf and 14 dpf. Bar, 500 μm. (B) Immunohistochemical OPG staining of the fracture sites in each group at 7 dpf and 14 dpf. Bar, 50 μm. (C) Immunohistochemical OPG staining quantitative analysis at 7 dpf and 14 dpf. Results are shown as mean ± SEM (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001. (D) Immunofluorescence staining of tibial fracture site (Pax7 + Sca1) at 14 dpf. Bar, 50 μm. (E) Immunofluorescence staining of tibial fracture site (Pax7 + BrdU) at 14 dpf. Bar, 50 μm. (F) Immunofluorescence staining analysis at 14 dpf. Results are shown as mean ± SEM (n = 6). ***P < 0.001
Double immunofluorescence staining revealed co-expression of Pax7 and Sca1 at tibial injury sites, suggesting that HD promoted SMSC expression by 14 dpf. In the TM-Vehicle group, only Pax7 expression was observed, indicating that HD upregulated SMSCs in damaged muscles at the early stage, though not surpassing the Con-Vehicle group (Fig. 5D). Additionally, Pax7 and BrdU double staining showed that HD significantly enhanced SMSC proliferation at 14 dpf compared to the TM-Vehicle group, achieving similar effects to the Con-Vehicle group (***P < 0.001; Fig. 5E-5F). These findings suggested that β-catenin is a critical signaling pathway through which HD treatment promotes SMSC growth and division in OPF, although other potential pathways may also contribute and require further investigation.
Active compound analysis by herbal decoction
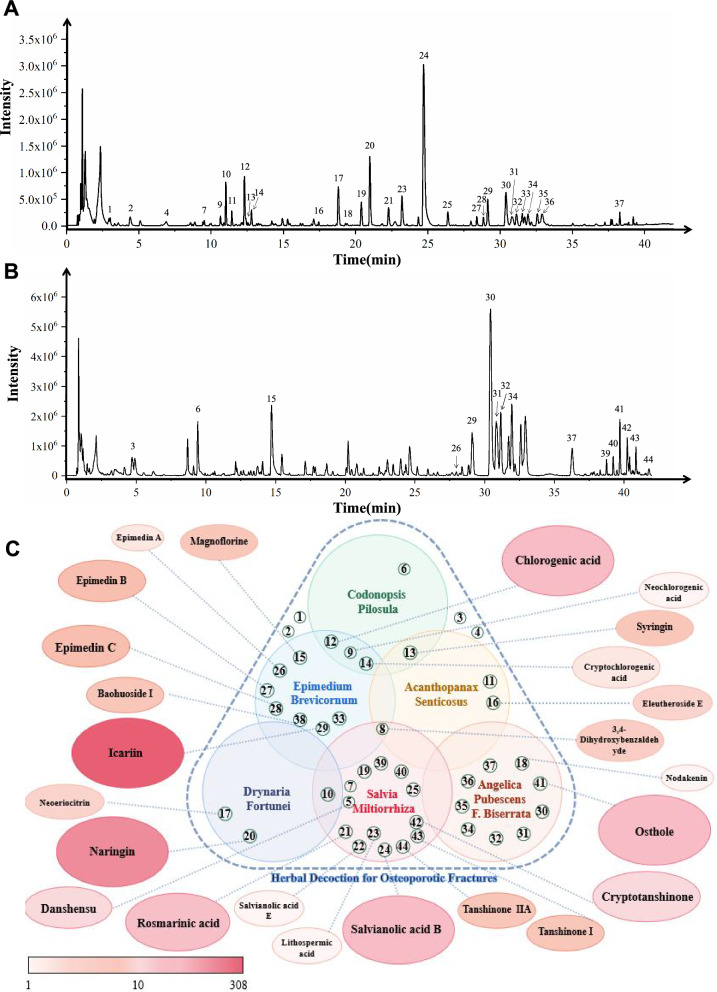
Active compound analysis of HD was conducted using HPLC-Q-TOF, producing ion chromatograms in negative (Fig. 6A) and positive modes (Fig. 6B). The analysis identified 44 peaks, of which 27 compounds were confirmed by comparison with standard compounds, while 17 were preliminarily identified (Table 2). Key compounds included:
Salvia Miltiorrhiza: Caffeic acid 3-O-β-D-glucoside, Salvianolic acid B, and 15 others.
Epimedium Brevicornum: Icariin, Magnoflorine, and 11 others.
Angelica Pubescens F. Biserrata: Osthole, Angenomalin, and 7 others.
Codonopsis Pilosula: Codonopsine, Chlorogenic acid, and 3 others.
Rhizoma Drynariae: Naringin, Neoeriocitrin, and 1 other.
Acanthopanax Senticosus: Eleutheroside B2, Eleutheroside E.
Fig. 6.
UPLC-Q-TOF/MS analysis of HD ingredients. (A) Representative ion chromatogram of ISTK in the negative mode. (B) Representative ion chromatogram of HD in the positive mode. (C) Hotspot maps of active compounds in orthopedic-related diseases were analyzed through the Pubmed search engine until March 2023
Table 2.
The mass information and source of identified compounds in herbal decoction by high-performance liquid chromatogAPBhy quadrupole time-of-flight mass spectrometry (HPLC–Q-TOF)
| No | Identification | Formula | Mass | Rt(min) | Error(ppm) | Classification |
|---|---|---|---|---|---|---|
| 1 | Uridine* | C9H12N2O6 | 244.07 | 3.00 | 1.1 | / |
| 2 | cGMP | C10H12N5O7P | 345.05 | 4.34 | 1.4 | / |
| 3 | Adenosine* | C10H13N5O4 | 267.10 | 4.70 | − 3.2 | / |
| 4 | Guanosine* | C10H13N5O5 | 283.09 | 6.91 | 0.8 | / |
| 5 | Danshensu* | C9H10O5 | 198.05 | 8.86 | 0.9 | SM |
| 6 | Codonopsine | C14H21NO4 | 267.15 | 9.41 | 1.3 | CP |
| 7 | Protocatechuicacid-3-O-glucoside | C13H16O9 | 316.08 | 9.43 | 1.5 | SM |
| 8 | 3,4-Dihydroxybenzaldehyde* | C7H6O3 | 138.03 | 10.28 | 0.8 | EB、SM、AS |
| 9 | Neochlorogenic acid* | C16H18O9 | 354.10 | 10.66 | 2.0 | CP、EB |
| 10 | Caffeic acid 3-O-β-D-glucoside | C15H18O9 | 342.10 | 11.00 | 3.9 | SM、DF |
| 11 | Eleutheroside B2 | C23H30O14 | 530.16 | 11.43 | 2.5 | AS |
| 12 | Chlorogenic acid* | C16H18O9 | 354.10 | 12.31 | 3.4 | CP、EB |
| 13 | Syringin* | C17H24O9 | 372.14 | 12.39 | 1.6 | CP、AS |
| 14 | Cryptochlorogenic acid* | C16H18O9 | 354.10 | 12.80 | 2.6 | CP、EB |
| 15 | Magnoflorine* | C20H24NO4 + | 342.17 | 14.82 | − 0.3 | EB |
| 16 | Eleutheroside E* | C34H46O18 | 742.27 | 17.45 | 1.8 | AS |
| 17 | Neoeriocitrin* | C27H32O15 | 596.17 | 18.78 | 3.5 | DF |
| 18 | Nodakenin* | C20H24O9 | 408.14 | 20.36 | 3.1 | APB |
| 19 | Salvianolic acid I | C27H22O12 | 538.11 | 20.40 | 2.2 | SM |
| 20 | Naringin* | C27H32O14 | 580.18 | 20.98 | 4.5 | DF |
| 21 | Rosmarinic acid* | C18H16O8 | 360.08 | 22.28 | 3.6 | SM |
| 22 | Salvianolic acid E* | C36H30O16 | 718.15 | 22.28 | 1.7 | SM |
| 23 | Lithospermic acid* | C27H22O12 | 538.11 | 23.19 | 1.3 | SM |
| 24 | Salvianolic acid B* | C36H30O16 | 718.15 | 24.71 | 2.5 | SM |
| 25 | Salvianolic acid Y* | C36H30O16 | 718.15 | 26.38 | 2.9 | SM |
| 26 | Epimedin A | C39H50O20 | 838.29 | 27.97 | 0.4 | EB |
| 27 | Epimedin B | C38H48O19 | 808.28 | 28.37 | 2.9 | EB |
| 28 | Epimedin C* | C39H50O19 | 822.29 | 28.82 | 1.8 | EB |
| 29 | Icariin* | C33H40O15 | 676.24 | 29.13 | 3.9 | EB |
| 30 | Angelol A* | C20H24O7 | 376.15 | 30.40 | 4.5 | APB |
| 31 | Angelol C | C20H26O7 | 378.17 | 30.84 | 0.1 | APB |
| 32 | Angelol E | C20H26O7 | 378.17 | 31.14 | 0.1 | APB |
| 33 | Anhydroicaritin-3-O-rhamnodide(1–2)-furanacid-7-O-glucoside | C39H48O19 | 820.28 | 31.53 | 3.0 | EB |
| 34 | Angelol B | C20H24O7 | 376.15 | 31.94 | 1.1 | APB |
| 35 | Angelol D | C20H24O7 | 376.15 | 32.58 | 0.0 | APB |
| 36 | Angelol F | C20H26O7 | 378.17 | 32.91 | 0.7 | APB |
| 37 | Angenomalin | C14H12O3 | 228.08 | 36.25 | − 1.5 | APB |
| 38 | Baohuoside I* | C27H30O10 | 514.18 | 38.28 | 3.9 | EB |
| 39 | 3-Hydroxytanshinone IIB | C19H18O5 | 326.12 | 38.73 | − 2.5 | SM |
| 40 | Isocryptotanshinone* | C19H20O3 | 296.14 | 39.21 | 0.5 | SM |
| 41 | Osthole* | C15H16O3 | 244.11 | 39.69 | − 3.4 | APB |
| 42 | Cryptotanshinone | C19H20O3 | 296.14 | 40.84 | 0.5 | SM |
| 43 | Tanshinone I* | C18H12O3 | 276.08 | 40.93 | − 1.6 | SM |
| 44 | Tanshinone II A* | C19H18O3 | 294.13 | 42.16 | 0.0 | SM |
*Identified with the reference compounds. CP: Codonopsis Pilosula. DF: Drynaria Fortunei. AS: Acanthopanax Senticosus. EB: Epimedium Brevicornum. SM: Salvia Miltiorrhiza. APB:Angelica Pubescens F. Biserrata
A PubMed literature review (March 2023) revealed extensive documentation of these compounds in bone-related research, particularly Icariin (308 articles), followed by Naringin (138), Chlorogenic acid (62), Osthole (51), and Salvianolic acid B (46). These findings emphasized the relevance of HD's active components in bone healing (Fig. 6C).
Pharmacological analysis and target identification
Web-based pharmacological analysis identified:
Codonopsis Pilosula: 17 components.
Drynaria Fortunei: 15 components.
Epimedium Brevicornum: 23 components.
Salvia Miltiorrhiza: 59 components.
Acanthopanax Senticosus: 17 components.
Angelica Pubescens F. Biserrata: 50 components.
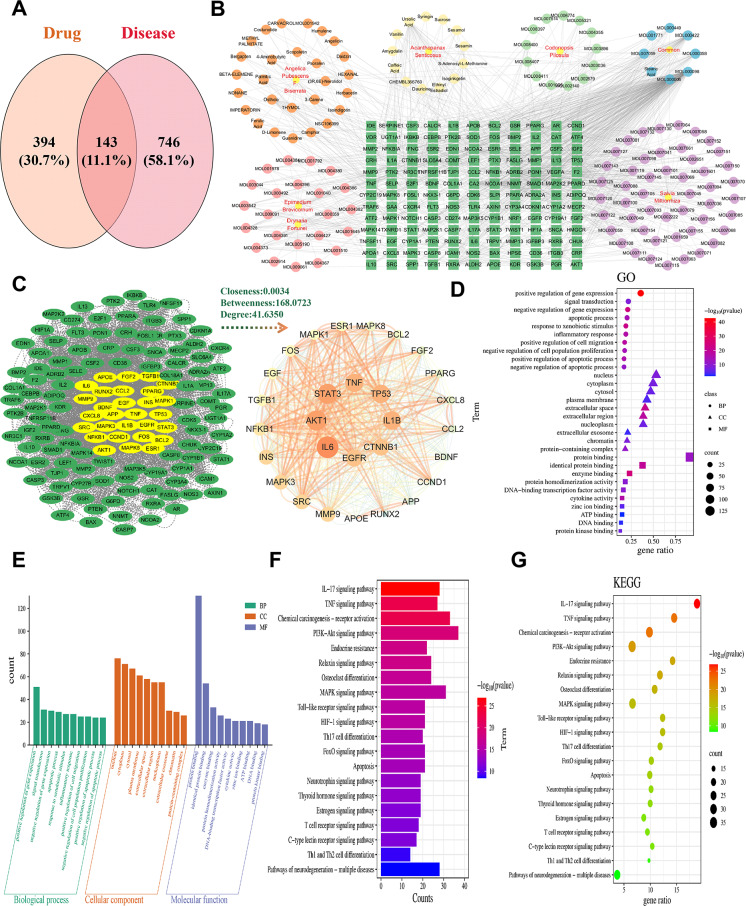
GeneCards and OMIM databases identified 889 osteoporotic fracture-related targets. After de-duplication, 143 intersecting drug-osteoporotic fracture targets were identified (Fig. 7A). A drug-ingredient-target network with 283 nodes and 1,084 edges highlighted quercetin, luteolin, kaempferol, ursolic acid, and beta-sitosterol as core ingredients (Fig. 7B). Protein–protein interaction networks revealed IL6, AKT1, STAT3, TNF, TP53, IL1B, EGFR, INS, SRC, and NFKB1 as core targets (Fig. 7C).
Fig. 7.
Network pharmacology analysis. (A)Venn diagram, after taking the intersection of the target of action of the drug with the target genes of OPF, 143 intersecting target genes of OPF and the drug were obtained as the interacting target genes for the drug treatment of OPF. (B) Drug-ingredient-target prediction results. Using the drug-ingredient-target data, and importing the files into Cytoscape 3.9.1 for graphing, the graph includes 283 nodes and 1084 edges. (C) The obtained 143 intersecting target genes were imported into the String (https://string-db.org/) database for protein–protein interaction prediction, with the species set to HomoSapiens and the confidence level set to 0.7. The network file was saved in TSV format, and the TSV file was imported into Cytoscape 3.7.2 software to draw the protein interaction network. (D) GO enrichment analysis. Drug-disease intersection genes were taken and GO gene function enrichment analysis was performed using the DAVID database, and a total of 638 GO entries were screened, with P < 0.01 as the standard screening. (E) 503 biological process (BP) major entries were significantly enriched for HD treatment of OPF; 48 entries were related to cellular components (CC); and 87 entries were related to molecular function (MF). (F) KEGG pathway enrichment analysis. (G) Pathway enrichment analysis was performed using the DAVID database, and a total of 172 pathways related to HD for osteoporotic fracture were enriched, and 150 pathways related to HD and treatment of osteoporotic fracture were screened according to **P < 0.01
GO and pathway enrichment analysis
GO gene function enrichment identified 638 entries (P < 0.01):
Biological Processes (503): Positive regulation of gene expression, signaling, apoptotic processes, and inflammatory responses.
Cellular Components (48): Nucleus, cytoplasm, extracellular exocytosis, chromatin.
Molecular Functions (87): Protein binding, DNA-binding transcription factor activity, cytokine activity (Fig. 7D and 7E).
Pathway enrichment analysis identified 172 pathways (P < 0.01), with 150 significantly associated with osteoporotic fractures, including IL-17, tumor necrosis factor, PI3K-Akt, osteoclast differentiation, MAPK, Toll-like receptor, and neurotrophic factor signaling pathways (Fig. 7F and 7G).
Discussion
This study demonstrated that HD prevented the significant reduction in bone volume and deterioration of bone microarchitecture in Pax7-CreERT2/+;β-cateninfx/fx cKO mice. HD modulated the β-catenin signaling pathway, promoted the migration of SMSCs to the fracture site, enhanced their differentiation into osteoblasts, and stimulated the activation of chondrocytes and osteoblasts at the OPF site. Additionally, HD improved spleen and stomach function, enhancing compound absorption, and facilitated the repair of surrounding muscles at the fracture site, collectively accelerating OPF healing. Interestingly, β-catenin knockout in SMSCs resulted in sarcopenia and osteopenia. HD stimulation improved fracture healing and remodeling of skeletal muscle fibers, indicating that HD regulated SMSCs to play a critical role in β-catenin-mediated osteosarcopenia fracture healing (Fig. 8).
Fig. 8.
Herbal compounds regulate skeletal muscle satellite cells and play a vital role in the healing of osteoporotic fractures mediated by β-catenin. When β-catenin is knocked out in SMSCs, sarcopenia and osteopenia are observed. Conversely, when β-catenin is promoted by HD, it promotes fracture healing and skeletal muscle fiber remodeling
Unlike previous research focused primarily on bone-specific mechanisms, this study emphasized the muscle changes surrounding fractures and found that HD not only promoted fracture healing by regulating osteogenesis and myogenesis of SMSCs through the β-catenin signaling pathway but also improved sarcopenia. This dual regulation of muscle and bone contributes to more efficient healing. Previous studies highlighted the significant potential of medicinal plants in osteoporosis prevention and treatment by regulating programmed cell death [25], underscoring their role as complementary and alternative therapies with reduced costs, minimal side effects, and broader applications. The specific composition and efficacy of HD further substantiate this potential.
For example, Icariin regulated osteogenesis and lipogenesis in stem cells through miR-23a-mediated pathway activation of Wnt/β-catenin, promoting osteogenesis while inhibiting lipogenesis [26]. Naringin@mesoporous bioactive glass nanoparticles (MBG) modified with β-cyclodextrin (CD-MBG) enhanced the transformation of macrophages to the M2 phenotype, creating an immune microenvironment conducive to osteogenesis while suppressing osteoclastogenesis [27]. Chlorogenic acid, identified as a potent inhibitor of Integrin-Binding Sialoprotein-receptor binding, effectively suppressed bone metastasis of estrogen-positive tumors, indicating its potential in preventing bone recurrence in such patients [28]. Additionally, our team previously demonstrated that Osthole mitigated osteoclast formation by stimulating β-catenin-OPG signaling activation [29]. Studies have also shown that SMSCs mediate osteoporotic fracture healing via β-catenin regulation, highlighting their therapeutic potential [17].
Moreover, Salvianolic acid B, encapsulated in hydrogel alongside bone marrow mesenchymal stem cells, significantly delayed disc degeneration [30]. Rosmarinic acid enhanced osteoblast differentiation, mineralization, and bone formation on titanium surfaces through the RANKL/RANK/OPG pathway [31, 32]. Combined treatment of Cryptotanshinone with Temsirolimus suppressed JAK/STAT, MAPK/ERK, and PI3K/Akt/mTOR pathways, inducing tumor cell apoptosis, and effectively combating osteosarcoma [33]. Danshensu demonstrated anti-osteogenic effects by suppressing JNK and ERK pathways, while also exhibiting significant anti-inflammatory properties [34, 35].
In contrast to previous research, this study is the first to use conditional knockout mice to assess the role of the traditional herbal remedy compound and elucidate its underlying mechanisms. The intervention with HD showed improved OPF healing in the TM-Decoction group; however, the results were not comparable to those observed in the Con-Vehicle group. This finding suggests that HD facilitates fracture healing through the β-catenin pathway and potentially other cellular mechanisms. Multiple studies have indicated that miR-122 hinders the proliferation and differentiation of osteoblasts in osteoporotic rats [36]. Bone morphogenetic proteins (BMPs) regulate normal satellite cell activity, balancing proliferation and differentiation before the activation of Noggin, which counteracts BMPs and promotes terminal differentiation [37, 38]. Additionally, it has been shown that ARC may inhibit osteoclastogenesis by inhibiting receptor activator of nuclear factor-κB ligand (RANKL), calcium signaling, and NFATc1. It also increases the activity of reactive oxygen species (ROS) enzymes via the Nrf2/Keap1/antioxidant response element pathway [39, 40]. Genetic disruption of Jagged-1/2 ligands and the Rbpj transcription factor within alpha Smooth Muscle Actin (αSMA)-expressing osteoblast progenitors revealed that Jagged-mediated Notch signaling in this lineage is essential for BMP-induced calvarial bone regeneration, as its inhibition impaired osteoblast progenitor expansion, reduced vascularization, and caused sustained inflammation [41]. And losartan treatment significantly improves bone morphology in OI mouse models by reducing Transforming Growth Factor β (TGFβ) levels, suppressing osteoclast activity, and increasing cortical and trabecular bone volume [42].
The accumulation of ROS in bone tissues due to oxidative stress associated with aging accelerates the senescence and impaired function of osteoblasts [43]. Furthermore, signaling pathways involving nuclear factor-kappa B, mitogen-activated protein kinases [44], protein kinase B [45], vitamin D [46], and the special AT-rich sequence-binding protein 2 (SATB2) [47], have been shown to facilitate the healing of osteoporotic fractures. Oxidative stress and inflammation also contribute to bone fracture healing [48], warranting further investigation.
Future studies should explore whether HD modulates the osteogenic differentiation of SMSCs through other critical osteogenic signaling pathways, such as BMPs, Smad4, and c-Jun. Additionally, follow-up studies to clarify the pharmacokinetics of HD are needed to establish clinical application guidelines. Given the numerous components identified within HD, optimizing the compound's composition ratio is essential. Future investigations should also include an analysis of additional signaling pathways using network pharmacology.
In summary, treatment with HD significantly improved the muscle wet weight coefficient in the osteosarcopenia futures model of Pax7-CreERT2/+; β-catenin fx/fx cKO mice. It enhanced muscle firmness and facilitated the formation of collagen fibers near fracture sites. Furthermore, HD stimulated the proliferative activity of SMSCs, promoting callus formation and accelerating fracture healing. These findings provide the first evidence that the HD, as a β-catenin agonist, not only promotes fracture healing by modulating the osteogenic and myogenic effects of SMSCs but also ameliorates sarcopenia. This study offers practical evidence supporting the formula as a promising therapeutic candidate for treating osteosarcopenic fractures.
Supplementary Information
Acknowledgements
Not applicable.
Declaration of AI and AI-assisted technologies in the writing process
All authors declare that AI and AI-assisted technologies were not used in the writing of this study.
Abbreviations
- ABHO
Alcian blue/hematoxylin & Orange G
- ARE
Antioxidant response element
- BMD
Bone mineral density
- BrdU
5-Bromo-2-deoxyUridine
- BSA
Bovine serum albumin
- BV/TV
Bone volume/total volume
- cKO
Conditional gene knockout
- dpf
Days post-fracture
- H&E
Hematoxylin and eosin
- HD
Herbal decoction
- MAPK
Mitogen-activated protein kinase
- micro-CT
Micro-computed tomography
- MyoD
Myogenic differentiation
- OPF
Osteoporotic fracture
- OPG
Osteoprotegerin
- Pax7
Paired box 7
- RANK
Receptor activator of nuclear factor-kB
- RANKL
RANK Ligand
- ROS
Reactive oxygen species
- Sca1
Stem cell antigen-1
- SMSCs
Skeletal muscle satellite cells
- Tb. N
Number of trabecular bone
- Tb. Th
Thickness of trabecular bone
- TCM
Traditional Chinese Medicine
- αSMA
Alpha Smooth Muscle Actin
- TGFβ
Transforming Growth Factor β
Author contributions
Z.X.J.: Investigation, Formal analysis, Resources, Methodology, Visualization, Funding acquisition, Writing—original draft. W.W.D.: Formal analysis, Methodology. Y.S.: Methodology. Y.J.Z.: Data curation. H.X.: Formal analysis. H.B.W.: Software. X.Q.W.: Visualization. X.G.: Data curation, Validation, Project administration. Y.L.: Data curation, Validation, Project administration. Q.S.: Conceptualization. D.Z.T.: Conceptualization, Funding acquisition, Resources, Writing—review and editing, Supervision. All authors reviewed the manuscript.
Funding
This study was sponsored by research grants from the National Natural Science Foundation of China (82305262, 81973883, 81730107), the National Key Research and Development Program of China (2018YFC1704300), the Shanghai Scientific Research Project of China (20S21902000), the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202202), and the Innovation Team of the Ministry of Education (IRT1270). The authors have no relevant financial disclosures.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiang Gao, Email: gaoxiang19710312@sina.com.
Yan Li, Email: 0001liyan@163.com.
Dezhi Tang, Email: dzt702@163.com.
References
- 1.Sayer AA, Cooper R, Arai H, Cawthon PM, Ntsama EM, Fielding RA, et al. Sarcopenia Nat Rev Dis Primers. 2024;10:68. [DOI] [PubMed] [Google Scholar]
- 2.Snyder S. Postmenopausal osteoporosis. N Engl J Med. 2024;390:675–6. [DOI] [PubMed] [Google Scholar]
- 3.Rapp K, Lamb SE, Roigk P, Becker C, Konnopka C, Konig HH, et al. Effect of an osteoporotic fracture prevention program on fracture incidence in routine care: a cluster-randomized trial. BMC Med. 2022;20:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mordenti M, Boarini M, Banchelli F, Antonioli D, Corsini S, Gnoli M, et al. Osteogenesis imperfecta: a cross-sectional study of skeletal and extraskeletal features in a large cohort of Italian patients. Front Endocrinol. 2023;14:1299232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii T, Murata K, Mun SH, Bae S, Lee YJ, Pannellini T, et al. MEF2C regulates osteoclastogenesis and pathologic bone resorption via c-FOS. Bone Res. 2021;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams J, Wilson N, Hurkmans E, Bakkers M, Balazova P, Baxter M, et al. 2019 EULAR points to consider for non-physician health professionals to prevent and manage fragility fractures in adults 50 years or older. Ann Rheum Dis. 2021;80:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010–2040. Osteoporos Int. 2015;26:2243–8. [DOI] [PubMed] [Google Scholar]
- 8.Gu Q, Koenig L, Mather RR, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472:3536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan D, Fan D, Yuan W. Cmtm3 suppresses bone formation and osteogenic differentiation of mesenchymal stem cells through inhibiting Erk1/2 and RUNX2 pathways. Genes Dis. 2021;8:882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smoak MM, Mikos AG. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater Today Bio. 2020;7:100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang WW, Sun XF, Tong HL, Wang YH, Li SF, Yan YQ, et al. Effect of differentiation on microRNA expression in bovine skeletal muscle satellite cells by deep sequencing. Cell Mol Biol Lett. 2016;21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelison D. “Known Unknowns”: Current Questions in Muscle Satellite Cell Biology. Curr Top Dev Biol. 2018;126:205–33. [DOI] [PubMed] [Google Scholar]
- 13.Corona BT, Greising SM. Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration. Biomaterials. 2016;104:238–46. [DOI] [PubMed] [Google Scholar]
- 14.En A, Watanabe K, Ayusawa D, Fujii M. The key role of a basic domain of histone H2B N-terminal tail in the action of 5-bromodeoxyuridine to induce cellular senescence. FEBS J. 2023;290:692–711. [DOI] [PubMed] [Google Scholar]
- 15.Montarras D, L’Honore A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–50. [DOI] [PubMed] [Google Scholar]
- 16.Xiang L, Liang C, Zhen-Yong K, Liang-Jun Y, Zhong-Liang D. BMP9-induced osteogenetic differentiation and bone formation of muscle-derived stem cells. J Biomed Biotechnol. 2012;2012:610952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Z, Da W, Zhao Y, Wang T, Xu H, Shu B, et al. Role of skeletal muscle satellite cells in the repair of osteoporotic fractures mediated by beta-catenin. J Cachexia Sarcopenia Muscle. 2022;13:1403–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander MS, Kawahara G, Motohashi N, Casar JC, Eisenberg I, Myers JA, et al. Microrna-199a is induced in dystrophic muscle and affects WNT signaling, cell proliferation, and myogenic differentiation. Cell Death Differ. 2013;20:1194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki A, Minamide R, Iwata J. WNT/β-catenin signaling plays a crucial role in myoblast fusion through regulation of nephrin expression during development. Development. 2018;145:v168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien S, Chidiac R, Angers S. Modulation of Wnt-beta-catenin signaling with antibodies: therapeutic opportunities and challenges. Trends Pharmacol Sci. 2023;44:354–65. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi S, Miyake T, Kelebeev J, McDermott JC. TAZ exhibits phase separation properties and interacts with Smad7 and beta-catenin to repress skeletal myogenesis. J Cell Sci. 2022;135:2. [DOI] [PubMed] [Google Scholar]
- 22.Yu D, Zhang S, Ma C, Huang S, Xu L, Liang J, et al. Ccl3 in the bone marrow microenvironment causes bone loss and bone marrow adiposity in aged mice. JCI Insight. 2023. 10.1172/jci.insight.159107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karner CM, Long F. Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci. 2017;74:1649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H, Wang B, Li J, Xie W, Mao Q, Li S, et al. Anti-DKK1 antibody promotes bone fracture healing through activation of beta-catenin signaling. Bone. 2015;71:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Li D, Chen R, Gao S, Xu Z, Li N. Cell death regulation: a new way for natural products to treat osteoporosis. Pharmacol Res. 2023;187:106635. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Jiang Y, Jia B, Wang Y, Li T. Icariin stimulates osteogenesis and suppresses adipogenesis of human bone mesenchymal stem cells via miR-23a-mediated activation of the Wnt/beta-catenin signaling pathway. Phytomedicine. 2021;85:153485. [DOI] [PubMed] [Google Scholar]
- 27.Mo Y, Zhao F, Lin Z, Cao X, Chen D, Chen X. Local delivery of naringin in beta-cyclodextrin modified mesoporous bioactive glass promotes bone regeneration: from anti-inflammatory to synergistic osteogenesis and osteoclastogenesis. Biomater Sci. 2022;10:1697–712. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Feng J, Lyu F, Xing F, Sharma S, Liu Y, et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat Commun. 2021;12:5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin ZX, Liao XY, Da WW, Zhao YJ, Li XF, Tang DZ. Osthole enhances the bone mass of senile osteoporosis and stimulates the expression of osteoprotegerin by activating beta-catenin signaling. Stem Cell Res Ther. 2021;12:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu J, Li C, Jin S, Ye Y, Fang Y, Xu P, et al. Salvianolic acid B combined with bone marrow mesenchymal stem cells piggybacked on HAMA hydrogel re-transplantation improves intervertebral disc degeneration. Front Bioeng Biotechnol. 2022;10:950625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong MJ, Lim DS, Kim SO, Park C, Choi YH, Jeong SJ. Effect of rosmarinic acid on differentiation and mineralization of MC3T3-E1 osteoblastic cells on titanium surface. Anim Cells Syst (Seoul). 2021;25:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayalar E, Goger F, Tas DG, Tok OE, Kucuk S. New bone-generative effect of Salvia officinalis L. in the expanded midpalatal suture : An in vivo and in vitro study. J Orofac Orthop. 2022;83:85–95. [DOI] [PubMed] [Google Scholar]
- 33.Vundavilli H, Datta A, Sima C, Hua J, Lopes R, Bittner M, et al. Anti-tumor effects of cryptotanshinone (C(19)H(20)O(3)) in human osteosarcoma cell lines. Biomed Pharmacother. 2022;150:112993. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Chen Z, Liao H, Zhong Y, Hua J, Su M, et al. Anti-osteogenic effect of Danshensu in Ankylosing Spondylitis: an in vitro study based on integrated network pharmacology. Front Pharmacol. 2021;12:772190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye T, Xiong D, Li Y, Gong S, Zhang L, Li B, et al. Inhibition of nuclear factor kappa B as a mechanism of Danshensu during Toll-like receptor 2-triggered inflammation in macrophages. Int Immunopharmacol. 2020;83:106419. [DOI] [PubMed] [Google Scholar]
- 36.Meng Y, Lin T, Jiang H, Zhang Z, Shu L, Yin J, et al. Mir-122 exerts inhibitory effects on osteoblast proliferation/differentiation in osteoporosis by activating the PCP4-mediated JNK pathway. Molecular Therapy - Nucleic Acids. 2020;20:345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X, Ji S, Li W, Yi B, Li H, Zhang H, et al. LncRNA H19 promotes the differentiation of bovine skeletal muscle satellite cells by suppressing Sirt1/FoxO1. Cell Mol Biol Lett. 2017;22:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao Y, Ni N, Huang L, Fan J, Wang H, He F, et al. Argonaute (AGO) proteins play an essential role in mediating BMP9-induced osteogenic signaling in mesenchymal stem cells (MSCs). Genes Dis. 2021;8:918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen D, Ye Z, Wang C, Wang Q, Wang H, Kuek V, et al. Arctiin abrogates osteoclastogenesis and bone resorption via suppressing RANKL-induced ROS and NFATc1 activation. Pharmacol Res. 2020;159:104944. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Wang C, Si J, Wang B, Zhang D, Ding D, et al. Melatonin up-regulates bone marrow mesenchymal stem cells osteogenic action but suppresses their mediated osteoclastogenesis via MT2 -inactivated NF-kappaB pathway. Br J Pharmacol. 2020;177:2106–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson HM, Buckles MA, Acevedo PK, Capobianco C, Nguyen DM, Kessell K, et al. Notch signaling in osteoblast progenitor cells is required for BMP-induced bone formation. Bone. 2025;194:117425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morita M, Arshad F, Quayle LA, George CN, Lefley DV, Kalajzic I, et al. Losartan alters osteoblast differentiation and increases bone mass through inhibition of TGFB signalling in vitro and in an OIM mouse model. Bone Rep. 2024;22:101795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HJ, Kim WJ, Shin HR, Yoon HI, Moon JI, Lee E, et al. ROS-induced PADI2 downregulation accelerates cellular senescence via the stimulation of SASP production and NFkappaB activation. Cell Mol Life Sci. 2022;79:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Bai J, Zhang W, Ge G, Wang Q, Liang X, et al. Protective effects of Punicalagin on osteoporosis by inhibiting osteoclastogenesis and inflammation via the NF-κB and MAPK pathways. Front Pharmacol. 2020. 10.3389/fphar.2020.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Liu Z, Du J, He J, Lin P, Amini B, et al. Thymidine phosphorylase exerts complex effects on bone resorption and formation in myeloma. Sci Transl Med. 2016;8:113r–353r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donato AA, Nesfeder J. Supplementation with vitamin D plus calcium reduces fracture risk; vitamin D alone does not. Ann Intern Med. 2020;172:C51. [DOI] [PubMed] [Google Scholar]
- 47.Huang X, Chen Q, Luo W, Pakvasa M, Zhang Y, Zheng L, et al. SATB2: a versatile transcriptional regulator of craniofacial and skeleton development, neurogenesis and tumorigenesis, and its applications in regenerative medicine. Genes Dis. 2022;9:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong G, Wan W, Liu X, Yin J. Apelin-13, a regulator of autophagy, apoptosis and inflammation in multifaceted bone protection. Int Immunopharmacol. 2023;117:109991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.