Abstract
Renowned for their distinctive aromas, terpenoid flavor compounds and their precursors are widely used in medicine, food, and the flavor and fragrance industries. Rapid advances in synthetic biology, including the modification of microbial chassis cells, the design of synthetic pathways for novel target products, and the integration of large-scale microbial fermentation, have enabled the development of microbial cell factories for the green and efficient production of terpenoid flavor compounds and their precursors, offering broader market potential. This review examines common biosynthetic mechanisms, recent progress in the field, and strategies for enhancing the biosynthetic efficiency of terpenoid flavor compounds and their precursors. This study aims to support the advancements of sustainable production technologies and promote industrial application within the flavor and fragrance sector.
Keywords: Terpenoids, Flavors and fragrances, Microbial cell factory, Biosynthetic strategy
Introduction
Terpenoids are a class of compounds formed through repetitive condensation reactions between isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (Fig. 1). Based on their isoprene unit count, they are classified as monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes (C40), and polyterpenes [1, 2]. These compounds are widely distributed in nature, exhibiting diverse biological activities and aromatic properties; hence, they are valuable in various applications, such as food and cosmetic fragrances [3]. Terpene aroma compounds, including nerol, linalool, and borneol, contribute distinct scents to fragrances [4, 5]. Precursor compounds undergo chemical transformations via processes such as high-temperature pyrolysis, thereby yielding aromatic components, such as abietadiene, sabinene, and ambergris alcohols.
Fig. 1.
Synthetic pathway of terpenoids. G6P Glucose-6-phosphate, G3P Glyceraldehyde-3-phosphate, PYR Pyruvate, DXP 1-Deoxy-D-xylulose 5-phosphate, MEP 2-C-Methyl-D-erythritol-4-phosphate, CDP-ME 4-diphosphocytidyl-2-C-methyl-D-erythritol, CDP-MEP 4-diphosphocytidyl-2-C-methyl-D-erythritol 2-phosphate, MEcPP 2-C-methyl-D-erythritol-2,4-cyclodiphosphate, HMB-PP 4-hydroxy-3-methylbut-2-enyl-diphosphate, Ac-COA Acetyl-CoA, MAL-COA Malonyl-CoA, AcAc-CoA Acetoaceyl-CoA, HMG-CoA 3-hydroxy-3-methylglutaryl-CoA, MVA Mevalonate, MVA-5P MVA-5-phosphate, MVA-PP MVA-5-diphosphate, MG-CoA 3-methylglutaconyl-CoA, MB-COA 3-methyl-2-butenal-COA, MB 3-methyl-2-butenal, Prenol 3-methyl-2-butene-1-ol, Isoprenol 3-methyl-3-butene-1-ol, IP Isopentenyl monophosphate, DMAP Dimethylallyl monophosphate, IPP Isopentenyl pyrophosphate, DMAPP Dimethylallyl pyrophosphate, GPP Geranyl diphosphate, FPP Farnesyl diphosphate synthase, GGPP Geranylgeranyl diphosphate, DXS 1-deoxy-D-xylulose 5-phosphate synthase, DXR 1-deoxy-D-xylulose 5-phosphate reductoisomerase, MCT MEP cytidylyltransferase, CMK CDP-ME kinase, MDS MEcPP synthase, HDS HMB-PP synthase, HDR HMB-PP reductase, ACAT Acetoacetyl-CoA transferase, HMGS HMG-CoA synthase, HMGR HMG-CoA reductase, MVK MVA kinase, PMVK Phosphomevalonate kinase, MVD Diphosphomevalonate decarboxylase, LiuC Enoyl-CoA hydratase, AibAB Glutaconyl-CoA decarboxylase, cbjALD Acyl-CoA reductase, YahK Alcohol dehydrogenase, ThiM Hydroxyethylthiazole kinase, IPK Isopentenyl phosphate kinase, CK Choline kinase, IDI Isopentenyl diphosphate isomerase, GPPS Geranyl diphosphate synthase, FPPS Farnesyl diphosphate synthase; GGPPS Geranylgeranyl diphosphate synthase
With evolving consumer preferences, the demand for natural terpenes is increasing. Currently, they are primarily obtained from plant extracts and chemical synthesis. However, these conventional methods face challenges, including low raw material content, high extraction costs, and environmental concerns, which limit their ability to meet market demand. In contrast, microbial biocatalysis offers considerable potential advantages. First, it is a streamlined technological process that significantly shortens the production cycle, thereby enhancing its cost-effectiveness and competitiveness in future markets. Second, it demonstrates superior environmental sustainability, closely aligning with the principles of green production [6–8].
Moreover, according to the flavoring regulations established by the U.S. FDA and the European Union (EC No. 1334/2008), aroma compounds produced via microbial fermentation using natural raw materials as substrates can be classified as “natural flavors” if they are chemically identical to those naturally present in the source materials and recognized as such by authoritative regulatory bodies. This classification not only meets consumer demand for “natural” products but also plays a pivotal role in facilitating market expansion and increasing the added value of flavoring products [9]. With advances in synthetic biology, various production technologies have significantly enhanced the capacity of micro-organisms to synthesize terpenoid compounds. For example, Meadows et al. used metabolic engineering techniques to optimize the central carbon metabolism pathway of Saccharomyces cerevisiae. This effort led to the development of a genetically modified strain. In a 200,000-L industrial fermenter, this strain successfully achieved a β-farnesene production level of 130 g/L, with a production efficiency of 2.24 g/L/h. These results demonstrated the effectiveness of the engineered strain and had a significant impact on reducing production costs [10]. However, traditional production methods for most terpenoid flavor compounds still offer a cost advantage, and biosynthetic approaches have not yet fully replaced conventional routes. Techno-economic analyses of microbial terpenoid production have consistently shown that productivity (i.e., production rate) and yield (i.e., total output) are the primary determinants of production cost. Thus, increasing product concentration is essential for achieving commercial viability [11, 12].
Researchers have used various hosts, including Escherichia coli, S. cerevisiae, and Yarrowia lipolytica, as chassis organisms to construct efficient microbial cell factories for ecofriendly terpenoid synthesis [13–16]. E. coli is a widely used bioengineering micro-organism. It offers advantages, including facile genetic modification, rapid growth, and high-density cultivation, making it particularly useful for synthesizing small-molecule terpenoids, such as linalool and limonene [17]. However, the biosynthesis of oxygenated terpenoids such as nootkatone is strictly dependent on the activity of cytochrome P450 (CYP) enzymes. These enzymes act as rate-limiting catalysts in terpenoid pathways and face inherent obstacles for efficient functional expression in prokaryotic systems [18]. In contrast, yeast systems (e.g., S. cerevisiae) provide a robust cellular milieu for P450 activity through eukaryotic organelles (e.g., endoplasmic reticulum), dedicated chaperone networks, and endogenous redox partner systems that facilitate proper folding, post-translational modification, membrane integration, and catalytic functionality [19, 20]. Therefore, yeast is the preferred chassis for terpenoid biosynthesis involving P450-dependent reactions. Overall, selecting an appropriate host organism is important for efficient terpenoid biosynthesis [21].
This review examines recent advances in the biosynthesis of key terpenoid flavor compounds and their precursors using E. coli and yeast as chassis organisms. This study also summarizes methodologies and strategies to enhance biosynthetic efficiency and promote green synthesis routes for terpenoid flavor compounds and their precursors, thereby supporting their future application and competitiveness within the flavor and fragrance industry.
Engineering E. coli for terpenoid flavor compound and precursor production
Escherichia coli offers several advantages for terpenoid synthesis. Its growth cycle is markedly shorter than that of eukaryotes, including yeast, allowing it to rapidly reach high cell density and improve synthesis efficiency [22]. Furthermore, E. coli is highly amenable to genetic modification. Numerous metabolic engineering tools, including plasmids, promoters, and regulatory elements, have been developed for E. coli to regulate key enzymes in terpenoid synthesis pathways [23]. Its high carbon utilization efficiency, simple fermentation requirements, and cost-effective cultivation further enhance the viability of E. coli as a host for terpenoid biosynthesis [24, 25].
In this section, we summarize the yields of terpenoid flavor compounds and their precursors synthesized through E. coli recently (Table 1). Notably, the biosynthesis of several compounds has reached levels suitable for industrial applications. Overall, E. coli has considerable potential for terpenoid biosynthesis through genetic engineering and metabolic pathway optimization.
Table 1.
Synthesis levels and aroma profiles of terpenoid flavor compounds and fragrance precursors in E. coli
| Product | Chemical formula | Titers (g·L−1) | Scale | Productivity (g·L−1·h−1) |
Aroma description | References |
|---|---|---|---|---|---|---|
| Nerol | 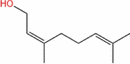 |
0.97 | shake flask | 0.012 | Fresh, sweet orange blossom and rose fragrance, with fruity notes reminiscent of raspberries; sweet with a hint of bitterness | [27] |
| Geraniol | 2.12 | shake flask | 0.044 | Resembling the scent of roses; a lingering fragrance with a subtle bitterness | [29] | |
| Limonene | 7.30 | 3.1-L fed batch | 0.15 | Fresh tangerine-lemon fruit scent with sweet green acidity; a light aroma | [33] | |
| Farnesol | 0.53 | shake flask | 0.011 | Warm floral scent, complemented by a subtle sweetness and a hint of fruitiness | [48] | |
| Linalool | 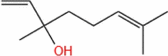 |
4.16 | 2-L fed batch | 0.035 | Lily of the valley scent, with lilac and rose flowers; a soft, light, lasting aroma | [51] |
| α-Pinene |  |
1.26 | 1.5 mL eppendorf tubes | 0.10 | Pure piney, woody and turpentine-like aroma | [42] |
| Borneol |  |
87.80 mg·L−1 | shake flask | 0.77 mg·L−1·h−1 | Intense fresh camphor aroma, with subtle woody and herbal undertones | [27] |
| (R)‐( +)‐Perillyl alcohol | 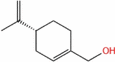 |
0.45 | 5-L fed batch | 1.71 mg·L−1·h−1 | Fresh, subtly sweet herbal aroma, complemented by the cool, distinctive fragrances of perilla leaves and mint | [150] |
| Myrcene | 1.25 | – | 0.014 | Delightful light balsamic aroma, sweet, fruity and fresh citrus notes | [151] | |
| (− )-α-Bisabolol |  |
23.40 | 1-L fed batch | 0.24 | Soft floral notes similar to chamomile or freesia, light floral | [152] |
| τ-Cadinol |  |
133.50 mg·L−1 | shake flask | 0.0037 | Rich woody notes, complemented by an earthy aroma, with warm herbs, and a subtle spicy undertone | [153] |
| α-Santalene | 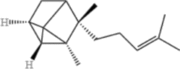 |
2.92 | 1.3-L fed batch | 0.48 mg·L−1·h−1 | Sweet, mild, and woody fragrance, a pleasant and long-lasting aroma | [77] |
| Nerolidol | 16.00 | 5-L fed batch | 0.17 | Subtle floral fragrance, reminiscent of roses and apples, characterized by sweetness, freshness, and longevity | [47] | |
| β-Elemene |  |
3.52 | 4-L fed batch | – | Spicy and woody scent | [154] |
| Patchoulol | 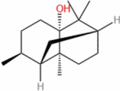 |
0.97 | 5-L fed batch | 0.0083 | Intense resinous aroma and herbal fragrance with minty, woody base notes | [155] |
| β‑Caryophyllene |  |
5.14 | 2-L fed batch | 0.080 | Distinctive spicy, woody, and clove-like aroma | [84] |
| Geranyllinalool | 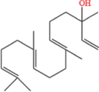 |
2.06 | 5-L fed batch | 0.014 | Sweet and fresh floral aroma, similar to lily of the valley | [54] |
| cis-Abienol | 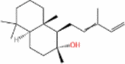 |
1.38 | 1.3-L fed batch | 0.012 | Oxidative degradation produces amber-like compounds with a unique fragrance | [58] |
| Ambrein | 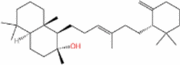 |
2.60 mg·L−1 | shake flask | 0.054 mg·L−1·h−1 | Oxidation and degradation form aromatic compounds, e.g., ambroxide, with a floral and vegetable oil-like fragrance | [62] |
Terpenoid flavor compounds
Nerol
Nerol is a monoterpene with a floral fragrance and a complex synthetic pathway. Recent studies have focused on optimizing substrate carbon flux regulation and improving the catalytic efficiency of nerol synthase (GmNES) to enhance yield and efficiency. Zong et al. used glucose as the carbon source and co-expressed truncated neryl diphosphate synthase and GmNES to achieve a nerol yield of 0.053 mg/L [26]. By overexpressing genes in the mevalonate (MVA) pathway to boost IPP and DMAPP supply and increasing carbon flux toward nerol synthesis via ERG10 overexpression, they achieved a~30-fold yield increase, reaching 1.6 mg/L in shake flask cultures. Lei et al. engineered E. coli using metabolic and protein engineering strategies for de novo nerol synthesis [27]. They screened 11 endogenous and heterologous phosphatases and replaced GmNES, finding that the endogenous hydrolysis enzyme NudJ in E. coli produced a higher nerol yield (261.88 mg/L). Shake flask fermentation further increased the yield to 966.55 mg/L.
Geraniol
Geraniol, a key fragrance component, is widely used in the food, cosmetics, and perfume industries. Its biosynthetic pathway involves two essential enzymes: geranyl diphosphate synthase (GPPS) and geraniol synthase (GES). Recent research has substantially improved geraniol synthesis through metabolic pathway engineering. Liu et al. used GPPS from Abies grandis and GES from Ocimum basilicum to synthesize geraniol in E. coli via the MVA pathway [28]. The addition of isopropyl myristate reduced geraniol volatilization and inhibited its conversion to geranyl acetate. Their study also revealed that E. coli acetylesterase converts geranyl acetate to geraniol, increasing the yield to 2.0 g/L through acetylesterase overexpression and starvation strategies. Wang et al. screened GPPS and GES, developed an E. coli platform strain for geraniol production, and markedly improved its yield [29]. Using fusion tag evolution engineering to optimize GES expression and increase metabolic flux from GPP to geraniol, they achieved a geraniol titer of 2124.1 mg/L through shake flask fermentation. Shukal et al. identified a novel enzyme RhNUDX1, derived from roses as an alternative to conventional GES. This approach effectively circumvented the limitations associated with plant-derived enzymes, such as protein precipitation and low catalytic activity, and achieved a geraniol titer of 0.91 g/L in shake flasks. This study proposes a novel alternative biosynthetic pathway for geraniol [30].
Limonene
Limonene, a monoterpene with a citrus aroma, is widely used in fragrances, cleaning products, and pharmaceuticals. Its biosynthesis requires multiple enzymes, particularly limonene synthetase (LS). Enhancing LS expression through genetic engineering is key to increasing limonene production. In addition, using nontoxic organic solvents to mitigate limonene’s inhibitory effects on micro-organisms and optimizing the MVA pathway are crucial strategies for boosting biosynthetic yield [31]. Willrodt et al. heterologously introduced the S. cerevisiae MVA pathway into E. coli using GPPS from A. grandis, and optimized and truncated LS codons from Mentha spicata [32]. Using glycerol as the sole carbon source, they obtained 2.7 g/L limonene in a 3.1-L fermenter. Rolf et al. optimized MVA pathway-related genes and LS from Mentha haplocalyx in E. coli, producing strain pJBEI-6410 [33]. During fermentation, they added a nontoxic organic solvent, diisononyl phthalate, to a 3.1-L fermenter. Using glycerol as the sole carbon source, they achieved a final limonene yield of 3.6 g/L.
α-Pinene
α-Pinene is a prevalent bicyclic monoterpene with notable anti-inflammatory and neuroprotective properties. It is synthesized via the MVA or MEP pathway, resulting in the formation of GPP, which is subsequently cyclized by α-pinene synthase (PS) [34, 35]. Bokinsky et al. first demonstrated microbial α-pinene production in E. coli by heterologously expressing the MVA pathway, GPPS, and PS, achieving a titer of 1.7 mg/L [36]. Subsequently, Yang et al. engineered E. coli strain YJM28 with a hybrid MVA pathway, GPPS2, and PS (Pt30), yielding a titer of 0.97 g/L under fed-batch fermentation conditions [37]. Further enhancements included the construction of GPPS–PS fusion proteins to ameliorate GPP feedback inhibition using computational enzyme engineering techniques, such as truncations and site-directed mutagenesis, and the development of modular E. coli coculture systems to distribute the metabolic burden [38–41]. The highest reported α-pinene titer to date, 1.26 g/L, was obtained using a cell-free biosynthesis system optimized for lysate composition and enzyme ratios [42]. However, α-pinene exhibits significant toxicity toward E. coli, and the limitations in precursor supply efficiency and the stability of the MVA/MEP pathways are challenges for large-scale production [43]. Future efforts should integrate host tolerance engineering and advanced enzyme evolution techniques to achieve sustainable and industrial-scale biosynthesis of α-Pinene [35].
Nerolidol
Nerolidol, a sesquiterpene compound, emits distinct floral and woody scents. It has potent antioxidant and antibacterial properties. Thus, it is widely used in cosmetics, personal care products, and other commercial products [44–46]. Tan et al. engineered E. coli as a chassis organism to progressively enhance its synthetic yield through a systematic engineering approach [47]. They initially screened seven distinct nerolidol synthase (LNS) enzymes from different sources and ultimately identified FaLNS from strawberry as the most catalytically active variant. Subsequently, promoter engineering was used to regulate the expression of the MVA pathway modules, knocked out byproduct synthesis genes (ΔldhA, ΔpflB, etc.) to block competing pathways, and optimized precursor supply through a glucose–glycerol–lactose mixed carbon source to achieve a nerolidol yield of 3.3 g/L in shake flasks (carbon conversion rate: 26.2%, > 90% of theoretical yield). Finally, using a two-phase extraction fed-batch fermentation process (with the addition of dodecane as the organic phase), 16 g/L of nerolidol was obtained in a 5-L bioreactor (carbon yield of 9%). This study established a new record for the highest microbial production of nerolidol, and its efficient carbon conversion strategy provides a foundation for low-cost industrial production.
Farnesol
Farnesol, a sesquiterpene alcohol, has considerable industrial value owing to its applications in perfumes, medicines, and biofuels. In micro-organisms, farnesyl diphosphate (FPP) can be hydrolyzed using phosphatase enzymes to produce farnesol. Wang et al. first heterologously introduced the MVA pathway and screened phosphatase genes upregulated during FPP accumulation [48]. The phosphatases YbjG and PgpB exhibited substantial FPP hydrolysis activity. In subsequent experiments, they demonstrated that overexpressing ispA–PgpB or ispA–YbjG markedly increased farnesol production. Using the engineered strain, they achieved a farnesol yield of 526.1 mg/L after 48 h of shake flask fermentation, which was 7.1-fold higher than that in the control group.
Linalool
Linalool, a floral monoterpenoid compound, is produced via linalool synthase (LIS). Improving LIS catalytic efficiency or optimizing its expression levels are key strategies for increasing linalool production [49]. Wu et al. used ScLIS from Streptomyces clavuligerus and enhanced its expression by modifying the ribosome binding site (RBS) [50]. They then used protein scaffolds to spatially colocalize EcIDI, AgGPPS, and ScLIS, thereby improving the catalytic efficiency of the cascade. By optimizing the fermentation conditions, a linalool yield of 1523 mg/L was achieved. Linalool production can be further enhanced by increasing acetyl-CoA supply, reducing byproduct accumulation, and improving substrate utilization through metabolic engineering. Wang et al. introduced the MVA pathway into E. coli, coupled with GPPS and MaLIS from Mentha aquatica. They optimized RBS and performed site-specific mutations to boost linalool synthesis. To minimize pyruvate loss and acetate accumulation, an engineered NADP + -dependent pyruvate dehydrogenase complex and a phosphoketolase bypass pathway were constructed, yielding 4160 mg/L of linalool [51].
Nootkatone
Nootkatone, a natural sesquiterpene with a citrus aroma, is widely used in flavorings and health supplements. Valencene synthase from Citrus sinensis can be efficiently expressed in E. coli, facilitating its use in nootkatone synthesis [52]. Girhard et al. screened 120 cytochrome P450 enzymes from various bacterial sources and selected E. coli as the chassis organism for the coexpression of CYP109B1 from Bacillus subtilis, putidaredoxin from Pseudomonas putida, and its reductase PdR. Consequently, they achieved a nootkatone yield of 120 mg/L [53].
Geranyllinalool
Geranyllinalool, a sesquiterpenoid compound synthesized from GPP and linalool, possesses a distinctive rose-like fragrance. Chang et al. engineered an artificial isoprenol utilization pathway (IUP) in E. coli to convert isoprenol into geranyllinalool via a two-step enzymatic process [54]. They screened geranyllinalool synthases from various sources and identified the enzyme from Nicotiana attenuata as the most efficient, achieving a yield of 274.78 mg/L geranyllinalool. Subsequently, they systematically investigated the effects of carbon source, isopropyl β-D-1-thiogalactopyranoside concentration, and isoprenol addition on product yield. Based on these findings, they scaled-up production in a 5-L fermenter. They achieved a final titer of 2.06 g/L geranyllinalool through fed-batch fermentation.
Sclareol
Sclareol is a natural diterpenoid primarily isolated from Salvia sclarea and is the major constituent of its essential oil. Schalk et al. used a functional genomics strategy to identify and characterize two previously unknown diterpene synthases, SsLPS and SsScS, from a sage flower cDNA library [55]. To establish a microbial biosynthesis process for sclareol, they introduced the MVA pathway into E. coli and overexpressed ERG20 to increase intracellular FPP levels, thereby increasing GGPP availability, which is a key precursor for diterpene synthesis. By coexpressing the SsLPS and SsScS enzymes, a yield of 418 mg/L was achieved in shake flask cultures. Moreover, when a two-phase fed-batch fermentation system containing dodecane as the organic phase was implemented in a 5-L bioreactor, the final titer of sclareol reached 1.5 g/L. This study provided the first experimental evidence for the dual-enzyme biosynthetic mechanism responsible for sclareol production in sage, laying the foundation for its industrial-scale microbial synthesis.
Flavor precursors
Cis-abienol
Cis-abienol is a natural diterpenoid that is predominantly found in the essential oils of pine and fir. Research on cis-abienol biosynthesis has focused on optimizing metabolic pathways and identifying key enzymes, such as cis-abienol synthase and abienol synthase [56]. Li et al. engineered the 1-deoxy-D-xylulose 5-phosphate (DXP) and MVA pathways in E. coli, which markedly increased cis-abienol production [57]. Screening results showed that cis-abienol synthase from Abies balsamea and abienol synthase from Salvia sclarea exhibited high catalytic activity. Using isopropyl myristate as a solvent in a fed-batch bioreactor, the final cis-abienol yield reached 634.7 mg/L. Zhang et al. introduced IUP into E. coli to enhance DMAPP supply by screening and optimizing ethanol kinases and isoprenyl phosphate kinases [58]. They further knocked out the aphA and yqaB genes to construct strain BD203 with double phosphatase deletion, further increasing DMAPP accumulation. After 112 h of fermentation in a 1.3-L fermenter, the engineered strain produced 1375.7 mg/L of cis-abienol.
Cembratriene-diol
Cembratriene-diol (CBT-diol) is a diterpenoid that is degraded into solanone, norsolanedione, and solanifuran. It is initially synthesized as cembratrien-ol (CBT-ol) from GGPP via cembratrien-ol synthase (CBTS) and is then oxidized by cytochrome P450 hydroxylase [59]. Schrepfer et al. introduced Nicotiana tabacum CBTS gene into E. coli chassis cells to produce CBT-ol, leveraging DXP pathway overexpression [60]. After 5 days of fermentation in a 5-L fermenter, they achieved a final concentration of 120 mg/L CBT-ol. Furthermore, Wang et al. introduced the IUP pathway into E. coli, systematically knocked out multiple endogenous pyrophosphatase genes, and enhanced membrane storage capacity through cell membrane engineering. Ultimately, they achieved a CBT-ol yield of 2.87 g/L in a 5 L bioreactor, which is the highest reported to date [61]. To improve P450 solubility in E. coli, the researchers fused Taxus cuspidata t-P450 with tobacco n-CPR; however, only trace amounts of CBT-diol were detected in shake flask cultures.
Ambrein
Ambrein, a triterpenoid primarily found in ambergris, is highly valued in the fragrance industry for its distinctive aroma. Owing to the scarcity of natural ambrein, research has focused on biosynthesis to meet demand while minimizing environmental impact. Ambrein biosynthesis relies on squalene as a precursor, with current efforts centered on engineering micro-organisms to synthesize squalene for conversion into ambrein. Ke et al. developed a synthetic pathway for the production of squalene-derived ambrein in E. coli [62]. They integrated ScERG9 from S. cerevisiae, encoding squalene synthase (SQS), into the E. coli genome to enhance squalene supply. They then co-expressed a mutant squalene–hopene cyclase (SHC D377C), tetraisoprenyl-β-curcumene cyclase (BmeTC), and SQS in E. coli LKsb, achieving an ambrein yield of 2.6 mg/L.
Engineering yeasts for terpenoid flavor compound and precursor production
As eukaryote micro-organisms, yeasts possess specialized organelles, such as the endoplasmic reticulum, Golgi apparatus, and mitochondria, which enable them to perform intricate post-translational modifications [63]. This characteristic provides yeasts with a major advantage in synthesizing complex terpenoids, including β-carotene and lycopene [64, 65]. One of the key strategies in yeast-based terpenoid production is organelle compartmentalization, which involves anchoring key enzymes to specific organelles. This localized enzyme enrichment increases the concentration of intermediates, thereby enhancing synthesis efficiency. Furthermore, yeast metabolic networks exhibit greater stability, allowing sustained growth even in environments with high substrate and product concentrations, supporting continuous terpenoid production. In addition, yeasts offer several advantages for terpenoid biosynthesis: an ability to accommodate more complex enzymes, ease of achieving stable expression of multiple genes, and high chromosome integration efficiency [66]. Considering their structural complexity, metabolic flexibility, and robust industrial potential, yeasts serve as superior microbial platforms for terpenoid biosynthesis. Moreover, they provide essential technical support for the sustainable and highly efficient synthesis of terpenoids. Table 2 summarizes the advancement in yeast-based synthesis of terpenoid flavor components and precursors recently, along with their aromatic properties.
Table 2.
Synthesis levels and aroma profiles of terpenoid flavor compounds and fragrance precursors in yeast
| Product | Chemical formula | Host | Titers (g·L−1) | Scale | Productivity (g·L−1·h−1) |
Aroma description | References |
|---|---|---|---|---|---|---|---|
| α-Terpineol | 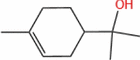 |
S. cerevisiae | 21.88 mg·L−1 | 5-L fed batch | 0.18 mg·L−1·h−1 | Lilac fragrance; delicate and fleeting | [70] |
| Geraniol | S. cerevisiae | 9.50 | shake flask | 0.079 | Resembling the scent of roses; a lingering fragrance with a subtle bitterness | [134] | |
| Borneol |  |
S. cerevisiae | 0.75 | shake flask | 4.50 mg·L−1·h−1 | Intense fresh camphor aroma, with subtle woody and herbal undertones | [156] |
| Citronellol | S. cerevisiae | 8.30 | 5-L fed batch | 0.072 | Fresh rose-like aroma with bitter notes | [157] | |
| β-Ionone |  |
Y. lipolytica | 0.98 | 3-L fed batch | 2.40 mg·L−1·h−1 | Sweet floral and woody aroma with creamy and fruity notes | [112] |
| Nerolidol | S. cerevisiae | 7.01 | 5-L fed batch | 0.049 | Subtle floral fragrance, reminiscent of roses and apples, characterized by sweetness, freshness, and longevity | [79] | |
| ( +)-Nootkatone |  |
S. cerevisiae | 2.39 | 3-L fed batch | 0.016 | Rich aroma of grapefruit peel, characterized by a fresh and vibrant scent, complemented by subtle bitter citrus notes, with warm woody and slightly earthy undertones | [93] |
| Valencene | 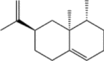 |
S. cerevisiae | 5.61 | 3-L fed batch | 0.032 | Rich citrus aroma with a woody undertone | [136] |
| (− )-Germacrene D |  |
S. cerevisiae | 7.90 | 5-L fed batch | 0.072 | Typical woody and spicy notes dominate, its odor is strong and well-diffused | [158] |
| Patchoulol | 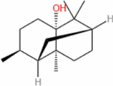 |
S. cerevisiae | 1.63 | 5-L fed batch | 0.018 | Intense resinous aroma and herbal fragrance with minty, woody base notes | [159] |
| α-Bisabolene |  |
S. cerevisiae | 18.60 | 5-L fed batch | 0.12 | Warm woody, citrusy, floral, fruity, and green notes with a sweet balsamic aroma | [160] |
| Santalol |  |
S. cerevisiae | 1.30 | 5-L fed batch | 0.018 | Creamy and woody aroma | [76] |
| β-Farnesene | S. cerevisiae | 130.00 | 20-m3 fed batch | 2.24 | Green and subtly sweet, featuring a pronounced honey-wax floral aroma with a distinct citrus undertone | [10] | |
| β-Caryophyllene | 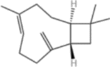 |
S. cerevisiae | 21.40 | 3-L fed batch | – | Distinctive spicy, woody, and clove-like aroma | [88] |
| Geranylgeraniol | S. cerevisiae | 6.33 | 5-L fed batch | 0.044 | Woody and herbal aromas, with mild floral and citrus notes | [91] | |
| Sclareol |  |
Y. lipolytica | 12.90 | 5-L fed batch | 0.054 | Ambergris-like scent; a subtle yet powerful aroma, with a lasting presence; endowing a vivid, harmonious, and enduring fragrance | [94] |
| Ambrein |  |
P. pastoris | 0.11 | 5-L fed batch | 1.40 mg·L−1·h−1 | Oxidation and degradation yield aromatic compounds, such as ambroxide, with a floral and vegetable oil-like fragrance | [96] |
| β-Carotene |  |
Y. lipolytica | 39.50 | 3-L fed batch | 0.17 | Degradation results in a distinct caramelized aroma, with a subtle earthy scent, possibly accompanied by a faint baking or smoky odor | [103] |
| Lutein |  |
S. cerevisiae | 595.30 μg·L−1 | shake flask | 6.20 μg·L−1·h−1 | Subtle grassy and vegetal aroma, complemented by a faint herbal fragrance and slight bitterness following degradation | [104] |
| Cembratriene-diol | 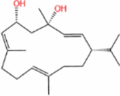 |
S. cerevisiae | 1.05 mg·L−1 | 3-L fed batch | 0.015 mg·L−1·h−1 | Roasted aroma, with a slightly sweet herbal scent and cocoa fragrance | [161] |
Terpenoid flavor compounds
Linalool
Insufficient precursor supply and low catalytic efficiency of LIS are major limitations in the efficient synthesis of linalool. Zhou et al. addressed these challenges using protein and metabolic engineering. First, they overexpressed the MVA pathway and an engineered GPPS mutant to enhance precursor availability [67]. They then screened LIS variants and identified a high-activity mutant, t67OMcLIS, which was further optimized through directed evolution. Using S. cerevisiae as the host strain, they achieved a yield of 53.14 mg/L linalool in shake flasks. Further optimization introduced a highly active LIS mutant, t67OMcLisE343D/E352H, and the ERG20F96W−N127W variant was assembled. In addition, endogenous expression of Erg20 was downregulated, which further increased linalool production in shake flasks to 80.9 mg/L [68]. Zhang et al. introduced IUP in S. cerevisiae to enhance IPP and DMAPP production [69]. They genomically integrated the S. cerevisiae genes IDI1 and ERG20F96W−N127W, and linalool synthesis was achieved using LIS (t26AaLS1) from Actinidia arguta. After optimizing substrate concentrations and using a bidirectional culture strategy, the final linalool yield was 142.88 mg/L after 72 h of shake flask fermentation.
α-Terpineol
α-Terpineol, a monoterpenoid alcohol with floral and citrus-like aromas, is synthesized through α-terpineol synthase (TPS). Improving TPS expression and catalytic efficiency is key to increasing production yields. Zhang et al. engineered an S. cerevisiae cell factory for α-terpineol production. By optimizing overexpression of the tHMG1, IDI1, and ERG20F96W−N127W genes, they increased α-terpineol titers to 0.83 mg/L [70]. To further enhance production, they fused ERG20F96W−N127W with a truncated α-terpineol synthase from Vitis vinifera using a GSGSGSGSGS linker, resulting in strain LCB07. Compared with the parental strain LCB03, the functional expression of the fusion protein in LCB07 increased α-terpineol yield by 2.87-fold, reaching 2.39 mg/L. However, attempts to boost FPP accumulation through ERG9 downregulation and LPP1/DPP1 deletion did not improve yields. Instead, ERG9 overexpression further elevated α-terpineol titers to 3.32 mg/L, forming strain LCB08. Using batch and fed-batch fermentation in a 5-L bioreactor, they achieved a final α-terpineol yield of 21.88 mg/L.
Geraniol
Geraniol synthesis in yeast is less efficient than that in E. coli, likely due to complex metabolic networks in yeast and competing pathways, e.g., the diversion of IPP and DMAPP into the ergosterol synthesis pathway. Although modulating ergosterol metabolism has improved production, competition from branch pathways continues to limit geraniol yields [71]. Zhao et al. used Candida glycerinogenes as a host and implemented dual-pathway engineering, achieving geraniol production of 858.4 mg/L when supplemented with 40 mM isopentenol [72]. To further reduce production costs, they developed a UPC2-mediated ergosterol feedback regulation system, which autonomously redirected carbon flux toward geraniol synthesis. By modifying the ergosterol promoter and optimizing transcription factor expression, they increased geraniol titers to 531.7 mg/L. To further enhance production, they engineered a xylose assimilation pathway in C. glycerinogenes and improved the pentose phosphate pathway, supporting efficient xylose metabolism. Using glucose as the carbon source, geraniol titers reached 1091.6 mg/L in a 5-L fermenter [73].
α-Santalene
α-Santalene, an essential sesquiterpene widely used in fragrances, has been subject to major improvements in yeast-based metabolic engineering [74]. Scalcinati replaced the native promoter of SQS (ERG9) with HXT1, which efficiently regulated ERG9 expression and redirected carbon flux toward α-santalene synthesis [75]. They also knocked out the LPP1 and DPP1 genes, which are involved in competing metabolic pathways, and overexpressed ERG20, achieving an α-santalene yield of 92 mg/L, representing a 3.4-fold improvement over the initial strain. Zha et al. also substituted the ERG9 promoter with HXT1, but instead of deleting competing pathways, they overexpressed GAL4, a transcriptional activator of the GAL promoter, to enhance α-santalene synthesis [76]. By increasing the expression of yeast phosphoglucomutase, they further improved galactose uptake, leading to increased α-santalene production. Ultimately, by introducing the α-santalene synthase SaSS from Santalum album, they achieved a maximum yield of 1.3 g/L. Zhang et al. screened FPPS variants to improve precursor flux and performed site-directed mutagenesis on α-santalene synthase, enhancing soluble enzyme expression and optimizing downstream pathways [77]. Furthermore, a fusion tag was introduced to the mutant enzyme, achieving final α-santalene titers of 1292 mg/L in shake flasks and 2916 mg/L in fermenters.
Nerolidol
Multifaceted metabolic reprogramming strategies have been developed to overcome the inherent metabolic constraints of S. cerevisiae, in which endogenous FPP is predominantly channeled toward ergosterol biosynthesis, and address the limitations imposed by its complex regulatory network on nerolidol production. Qu et al. enhanced the metabolic flux of S. cerevisiae’s MVA pathway and implemented a dual-induction system using the GAL promoter to synthesize nerolidol [78]. Overexpressing the transcription factor HAC1 increased nerolidol yield to 497.0 mg/L in shake flasks. Li et al. constructed an S. cerevisiae cell factory for high nerolidol production [79]. They overexpressed the MVA pathway through the GAL promoter, knocked out the GAL80 gene to optimize the glucose response of the GAL regulatory systems, and integrated UPC2-1 at the GAL80 locus. In addition, they replaced the native ERG9 promoter with HXT1 (a glucose-sensitive promoter) to reduce FPP consumption, achieving a final yield of 7.01 g/L in a fermenter.
β-Caryophyllene
β-Caryophyllene is a bicyclic sesquiterpene that has a mild clove-like aroma. It is widely used in fragrances, flavors, pharmaceuticals, and cosmetics and is also considered a promising biofuel because of its high energy density [80–82]. Previous studies have demonstrated the microbial synthesis of β-caryophyllene; however, the yields have not yet reached the levels necessary for commercial viability [83–86]. Li et al. identified and cloned four β-caryophyllene synthase genes from the traditional Chinese medicinal plant Artemisia argyi, with AarTPS88 showing the highest catalytic activity [87]. Using S. cerevisiae as a host, they co-expressed ERG20 with AarTPS88 to enhance the flux of the precursor FPP. Using a metabolic engineering strategy in a 5-L bioreactor through fed-batch fermentation, the yield of β-caryophyllene reached 15.6 g/L. Zhang et al. adopted a system engineering approach to regulate the sterol biosynthesis pathway of S. cerevisiae, thereby significantly enhancing β-caryophyllene production [88]. The key gene promoters (ERG9, ERG1, ERG7, and ERG11) were replaced with the glucose-responsive promoter PHXT1 to redirect the carbon flux toward β-caryophyllene synthesis. Furthermore, the promoters of fatty acid synthesis genes (ACC1, FAS1, and FAS2) were substituted with PERG7 to minimize metabolic competition. Furthermore, the glucose-sensing pathway was fine-tuned by modulating the expression of the transcription factor Rgt1 and the protein kinase Tpk2. Finally, diploid fusion was applied to restore growth performance. The final engineered strain achieved a β-caryophyllene titer of 21.4 g/L in fed-batch fermentation. This study describes an efficient cell factory for the industrial production of β-caryophyllene and offers a strategy for the synthetic biological modification of other terpenoid compounds.
Geranylgeraniol
Geranylgeraniol (GGOH), a diterpenoid with mild floral and citrus aromas, is primarily derived from Bixa orellana. Advancements in GGOH synthesis have focused on constructing cell factories using yeast strains, including S. cerevisiae and Y. lipolytica. These efforts enhanced carbon flux toward GGOH by downregulating squalene biosynthesis and increasing the supply of precursors (e.g., IPP), among other strategies [89]. Wang et al. used Y. lipolytica as a host, enhancing GGOH synthesis by increasing FPP, GGPP, and acetyl-CoA availability while downregulating squalene biosynthesis through promoter engineering [90]. By optimizing the fermentation conditions and reducing reactive oxygen species production, they achieved a GGOH titer of 3346.67 mg/L in shake flasks. Further improvements introduced NADH-dependent HMG–CoA reductase from Silicibacter pomeroyi to enhance NADH utilization, along with the overexpression of PaGGPPs–ERG20, PaGGPPs–DPP1, and the MVA pathway as well as the downregulation of ERG9 expression. Using S. cerevisiae, they achieved a yield of 6.33 g/L GGOH in a bioreactor [91].
Nootkatone
Yeast is more suitable than E. coli for nootkatone synthesis owing to its superior P450 enzyme functionality. Its endoplasmic reticulum supports proper enzyme folding and cofactor availability, and native P450 expression enhances heterologous P450 stability and activity [92]. Liu et al. engineered S. cerevisiae using a high-throughput screening system based on 2,4-dinitrophenylhydrazine–carbonyl reactions to identify candidate P450 oxygenase (HPO) mutants [93]. Semi-rational engineering of HPO and P450 reductase increased catalytic efficiency by 2.54-fold with the optimal mutant HPO_M18. They further knocked out ERG9 and the transcription factor ROX1 to inhibit squalene synthesis and overexpressed key enzymes in the MVA pathway, namely, tHMG1 and ERG12. In addition, CnVS, HPO_M18, and ADHm were introduced. This resulted in a ( +)-nootkatone yield of 2.39 g/L through two-stage, fed-batch fermentation, the highest yield reported to date.
Sclareol
Strategic integration of enzyme engineering for catalytic optimization with metabolic network rewiring to augment precursor flux constitutes the predominant paradigm for high-titer sclareol biosynthesis in yeast-based platforms. Sun et al. engineered a biosynthetic sclareol production pathway in Y. lipolytica by evaluating multiple truncated and full-length sclareol synthases (Lpps), with the truncated tSsLpps exhibiting the highest catalytic efficiency [94]. To further enhance precursor availability and minimize substrate diffusion losses, they engineered a novel pathway and scaffold-free enzyme complex. This approach led to a final sclareol yield of 12.9 g/L after 240 h of fermentation. Cao et al. initially fused the diterpene synthases Lpps and TPS and incorporated maltose-binding protein to enhance the stability of the fusion protein. Next, they reconfigured the metabolic network to increase the availability of acetyl-CoA and GGPP while suppressing competing metabolic pathways. High-density fermentation in a 5-L bioreactor resulted in a final sclareol yield of 11.4 g/L. This study demonstrated the efficient microbial biosynthesis of sclareol and validated the feasibility of the theoretical framework known as “metabolic module adaptability.” These results provide a foundation for the rational design and engineering of high-performance microbial cell factories [95].
Flavor precursors
Ambrein
Yeast-based synthesis is well-suited for ambrein production. Moser et al. enhanced squalene accumulation in Pichia pastoris by downregulating squalene epoxidase (ERG1) and reducing metabolic flux toward ergosterol [96]. They engineered BmeTC D373C, a mutant with superior catalytic efficiency compared to the cascade reaction involving BmeTC D373C and BmeTC. Using strain PPIS1-ERG1 TC D373C, fermentation in a 5-L bioreactor over 74 h yielded > 100 mg/L ambrein. Lin et al. developed an engineered yeast strain for de novo ambrein production by optimizing the MVA pathway [97], overexpressing codon-optimized BmeTC and its mutant BmeTC Y167A/D373C, testing various promoters, and knocking out GAL80. However, the low catalytic efficiency of BmeTC remained a bottleneck. To overcome this, they modified the surface amino acids of BmeTC, generating the variant BmeTCK6A/Q9E/N454A. This resulted in a 457.4 mg/L ambrein yield, the highest reported in yeast to date.
β-Carotene
β-Carotene, a tetraterpenoid aromatic compound, is extensively used in various industries, including animal feed, food products, pharmaceuticals, and cosmetics [98–101]. Lin et al. developed a high-yield β-carotene-producing S. cerevisiae strain by enhancing the MVA pathway genes, modulating squalene synthesis, and deleting FLD1 to expand lipid droplet capacity, which resulted in a yield of 2.09 g/L β-carotene in a 5-L bioreactor [102]. MA et al. introduced two sources of phytoene desaturase and bifunctional phytoene synthase/lycopene cyclase [103]. They found that lycopene cyclase was inhibited by lycopene, creating a bottleneck in carotenoid biosynthesis. To overcome this, they developed two strategies that nearly eliminated lycopene-induced substrate inhibition and enhanced carotenoid production: (1) using phylogeny-based and structure-guided protein engineering, they generated mutants that eliminated substrate inhibition while maintaining enzyme activity, increasing β-carotene yield; and (2) establishing a GGPPS-mediated metabolic flux regulator to control lycopene formation, keeping intracellular lycopene levels below the “inhibition threshold” to prevent substrate inhibition and boost β-carotene yield. Ultimately, the engineered strain produced 39.5 g/L β-carotene in a fermenter.
Lutein
Lutein, a tetraterpenoid, has degradation products, such as β-ionone, that contribute to floral and fruity aromas, making it valuable in the fragrance industry. In lutein biosynthesis, lycopene undergoes asymmetric cyclization through lycopene cyclase to form α-carotene, which is hydroxylated by carotenoid hydroxylase to produce lutein. However, the preference of lycopene cyclase for symmetric cyclization, leading to β-carotene formation, creates a major bottleneck. To mitigate metabolic flux loss from competition in the β-cyclization branch, Bian et al. implemented a sequential expression strategy using two lycopene cyclases, regulated by the temperature-sensitive Gal4M9 protein [104]. This approach redirected metabolic flux toward α-carotene production. In addition, ε-cyclase was engineered and relocated to the plasma membrane to further optimize α-carotene synthesis. The pathway was enhanced by incorporating carotene hydroxylase CYP97A3 and Lut1 from Arabidopsis thaliana, with α-carotene hydroxylation efficiency improved by adjusting CYP97A3 copy numbers. These optimizations led to a lutein yield of 595.3 μg/L in yeast.
Strategies for enhancing terpenoid biosynthesis
Microbial cell factories offer a promising approach to terpenoid production given their short cycle, ease of operation, and low cost [105, 106]. This method also supports sustainability, high efficiency, enantiomeric purity, and scalability. Despite major progress in microbial fermentation, increasing terpenoid yield remains challenging owing to uncoordinated gene expression in metabolic pathways [107], low enzyme activity [108], and limited precursor availability [109]. With the rapid advancements in synthetic biology, a range of effective biosynthetic strategies have been developed to address these challenges. These approaches enhance precursor supply and optimize metabolic flux toward terpene biosynthesis [2], laying a solid foundation for improving the yield of terpenoid flavor compounds and aroma precursors.
Modular pathway engineering
Engineering micro-organisms for terpenoid synthesis requires regulating endogenous pathways and, in some cases, introducing exogenous pathways. These modifications can disrupt the metabolic flux, affecting target compound yields. Addressing these metabolic flux perturbations remains a key challenge [110]. This modular design breaks down complex pathways into distinct functional units, such as modules for upstream precursor supply, core scaffold synthesis, and downstream product modification, allowing hierarchical regulation and flexible adjustment of metabolic fluxes, which significantly improves the efficiency of terpene synthesis. Furthermore, modular design offers a rational solution by maintaining high carbon flux during synthesis of the C5 intermediate, isopentenyl pyrophosphate. By modifying downstream modules of the intermediate, various terpenoids can be efficiently produced, improving overall yield (Fig. 2A). For example, Zhang et al. developed a four-module apocarotenoid synthesis platform in E. coli, which consisted of the upstream MVA pathway (module 1), downstream MVA pathway (module 2), lycopene biosynthesis module (module 3), and apocarotenoid modification module (module 4) [111]. Using experimental design-assisted system pathway optimization, the metabolic flux among the modules was effectively balanced, resulting in a lycopene yield of 107 mg/L under shake flask conditions. By substituting different combinations of enzymes in module 4 (e.g., LCYe-CCD1 → crtY-CCD1 → crtY-BCDO), a “plug-and-play” strategy enabled the production of α-ionone (480 mg/L), β-ionone (500 mg/L), and retinol (33 mg/L). Lu et al. applied modular pathway engineering to enhance β-ionone synthesis in Y. lipolytica [112], dividing the biosynthetic pathway into three modules: the precursor acetyl-CoA synthesis module, the MVA pathway module, and the β-ionone synthesis pathway module. Through pathway design and assembly, they constructed a high-yield strain. Fed-batch fermentation in a 3-L fermenter produced a maximum β-ionone yield of 0.98 g/L. In conclusion, modular design enables the systematic coordination of multiple enzyme expressions and offers a versatile framework for enhancing the diversity of downstream products.
Fig. 2.
Strategies to enhance the biosynthetic yield of terpenoids. A Modular pathway engineering; B genomic editing (CRIPSR/Cas9/Cpf1); C enzyme engineering; D cascade catalysis based on protein scaffold; E organelle compartmentalization, MTS: mitochondrial targeting signaling peptide; F genetic screening based on machine learning; G adaptive evolution improves strain tolerance evolution; and H microbial coculture fermentation.
Genomic editing
CRISPR/Cas9, derived from the Type II CRISPR/Cas system, simplifies gene editing in micro-organisms, including those that are traditionally difficult to modify. It enables precise insertions, deletions, and mutations, facilitating metabolic pathway engineering in prokaryotic and eukaryotic systems. Thus, CRISPR/Cas9, in addition to variants, such as CRISPRi and CRISPRa, promotes increased biosynthesis of terpene flavor compounds (Fig. 2B).
Using CRISPR/Cas9 for microbial genome editing, metabolic networks can be modified through gene knockouts or insertions to redirect carbon flux toward target metabolites and enhance product yield. Eauclaire et al. used CRISPR/Cas9 to integrate 17 fragments into the S. cerevisiae genome, successfully constructing a complete β-carotene biosynthetic pathway [113]. In addition, CRISPR/Cas9-based approaches enable precise regulation of target genes to boost terpenoid synthesis. Lian et al. used CRISPRa to upregulate HMG1, a gene encoding a rate-limiting enzyme in the mevalonate pathway, and used CRISPRi to downregulate ERG9 in the β-carotene and sterol biosynthesis branch pathways [114]. They also knocked out the transcriptional regulatory factor gene ROX1, achieving a threefold increase in β-carotene synthesis. Zhang et al. further improved yields by overexpressing MVA pathway genes and introducing multiple copies of β-carotene biosynthesis genes via CRISPR/Cas9 [115]. Combined with optimized fermentation conditions, this approach resulted in a β-carotene yield of 4.5 g/L.
Enzyme engineering
In terpenoid biosynthesis, the low catalytic activity, poor specificity, and insufficient stability of key enzymes are major factors limiting yield. Strategies such as directed evolution, rational design, and semi-rational design are commonly used to construct mutant libraries and identify optimal enzymes. However, these methods require an in-depth understanding of protein structure and function and are often labor-intensive [116]. To overcome these limitations, efforts in recent years have focused on protein fusion and chemical modification as effective strategies for enhancing enzyme activity and increasing target product yields [117] (Fig. 2C, andD). Park et al. developed an innovative electron conduction strategy using the Photorhabdus luminescens crystal protein CipB as a scaffold [118]. By assembling multiple enzymes into functional protein crystalline inclusions, this approach facilitated direct enzyme–enzyme interactions and enabled efficient electron transfer between P450s and reductases. Applying this strategy in E. coli for lutein biosynthesis, they optimized the heme pathway and fermentation conditions, achieving a lutein yield of 218 mg/L within 2 days. Similarly, Kang et al. improved the efficiency of cascade reactions in micro-organisms using the spherical protein cage Mi3 [119]. Using E. coli as a host, they biosynthesized lycopene and used the protein cage system to anchor multiple pathway enzymes to the Mi3 surface. This strategy resulted in an 8.5-fold increase in lycopene production. Increasing product yields and ensuring cost-effective production are essential for widespread application.
Organelle compartmentalization and transporter engineering
In eukaryotic micro-organisms, such as S. cerevisiae, organelle compartmentalization has been used to enhance terpenoid synthesis by taking advantage of the distinct physicochemical conditions of specific organelles (Fig. 2E) [120, 121]. By anchoring key enzymes to organelles, such as the endoplasmic reticulum, mitochondria, and plasma membrane, local enzyme and intermediate concentrations are increased, improving synthesis efficiency. Several engineering strategies have been successfully implemented, such as endoplasmic reticulum engineering [122], lipid droplet engineering [123, 124], peroxisome engineering [125, 126], mitochondrial engineering [127], and plasma membrane engineering [128]. A major limitation in microbial terpenoid biosynthesis is the cytotoxicity of accumulated target products, which reduces cell viability and hinders efficient production [129]. Transporter engineering has emerged as a viable solution, involving the upregulation of ABC transporter expression and the development of artificial membrane vesicle trafficking systems (AMVTS) to facilitate the secretion of terpenoid compounds into the extracellular space [130, 131]. These approaches could also facilitate the recovery of the target products.
Matsumoto et al. hypothesized that the high concentration of acetyl-CoA in mitochondria could enhance carotenoid biosynthesis [132]. By relocating the carotenoid biosynthetic pathway to mitochondria, they achieved a 13.82-fold increase in carotenoid yield compared with that of cytoplasmic engineering strains. Dusseaux et al. found that the introduction of a heterologous MVA pathway, along with GPPS and monoterpene synthases, into peroxisomes enhanced the conversion of acetyl-CoA into valuable monoterpenoids. This strategy is more efficient than cytoplasmic production. If DMAPP, IPP, and GPP are not rapidly used within the peroxisome, they can be transported back to the cytoplasm through transporters, such as Pxmp2. This mechanism reduces intermediate loss and sustains a high local substrate concentration, thereby increasing the production yield [133]. Baker et al. reconstructed the MVA pathway in S. cerevisiae peroxisomes by implementing a dual-compartment fermentation strategy to coexpress it with the cytoplasmic pathway for geraniol production [134]. Peroxisomal capacity was directly correlated with synthesis efficiency. By overexpressing key genes and integrating machine learning approaches (Fig. 2F), they enhanced peroxisomal capacity by 1.37-fold, ultimately achieving a geraniol yield of 9.5 g/L under fed-batch conditions. Excessive intracellular terpenoid accumulation can impair cellular activity, thereby reducing biosynthetic efficiency, particularly for cytotoxic terpenoids. To address this, transport protein engineering strategies facilitate the secretion and recovery of target products while minimizing their adverse effects. Wu et al. developed the AMVTS in E. coli, using membrane lipids for the transport of hydrophobic molecules, effectively increasing hydrophobic terpenoid secretion [131]. Their study also identified lipid proteins, such as TolR and NlpI, as key regulators of outer membrane vesicle formation. Combining the AMVTS method with tolR and nlpI knockouts led to a 13.7% increase in β-carotene secretion.
Adaptive laboratory evolution
Adaptive laboratory evolution (ALE) is an undirected genomic modification strategy (Fig. 2G). Considering that certain terpenoids exhibit cytotoxicity, which limits synthesis efficiency, ALE can enhance the tolerance of chassis cells to high concentrations of these compounds. Niu et al. enhanced the tolerance and yield of E. coli to α-pinene using the ALE strategy. They subjected E. coli to atmospheric and room-temperature plasma mutagenesis treatment and gradually increased the concentration of α-pinene in the culture medium from 0.5% to 2.0% for adaptive selection. They obtained a strain that was tolerant of 2.0% α-pinene and increased its yield by 31% [135]. Zhu et al. used S. cerevisiae with mannitol as the carbon source to engineer a cell factory for efficiently producing valencene [136]. Wild-type S. cerevisiae cannot utilize mannitol; therefore, the researchers applied adaptive evolution to a previously engineered valencene-producing strain, yielding the mutant strain BN-91A, which could grow on mannitol-based media. Compared with glucose, mannitol tripled valencene production. Further optimization of precursor supply, substrate uptake, and cofactor regeneration led to a final yield of 161.1 mg/L in shake flasks and 5.61 g/L in a 3-L fermenter. Limonene’s cytotoxicity also poses a challenge for efficient biosynthesis. Li et al. used transcriptomics to identify eight genes in Y. lipolytica that improved tolerance to limonene [137]. Overexpression of YALI0F19492p increased limonene production eightfold, demonstrating that tolerance and evolutionary engineering can markedly enhance limonene yield and host strain robustness.
Bioderivatization
The enzymatic derivatization strategy effectively alleviates product toxicity and significantly improves synthesis efficiency [138, 139]. This approach involves the expression of specific transferases, such as alcohol acetyltransferases or glycosyltransferases, which catalyze the conversion of hydrophobic terpenoids, particularly toxic compounds, such as geraniol, into low-toxicity derivatives, such as acetates or glycosides. The target end-products can then be reverted to their original chemical forms through hydrolysis, which is an important step in the product recovery process [28]. For example, Priebe et al. developed a byproduct-free, whole-cell catalytic process for geraniol glycosylation and demonstrated that enzymatic detoxification can enhance the biosynthesis of terpenoids [140]. Wang et al. successfully converted cytotoxic geraniol into the less harmful geranyl acetate by expressing alcohol acetyltransferase from Rosa hybrida (RhAAT). This enabled host cells to sustain normal growth at geraniol-equivalent concentrations up to 700 mg/L [141]. Through systematic optimization of fusion protein design, amplification of AAT gene copy number, and glycerol concentration-controlled fed-batch fermentation, they achieved the highly selective production of geranyl acetate at 10.36 g/L in a 1.3-L bioreactor. This integrated strategy not only significantly reduced geraniol-induced toxicity but also improved carbon conversion efficiency to 0.126 g/g glycerol. Similarly, Shukal et al. reported in situ acetylation of geraniol to geranyl acetate by coexpressing RhNUDX1 and RhAAT1 [30]. A final geranyl acetate titer of 19.0 g/L was obtained from a 5-L bioreactor after systematic fermentation optimization.
Coculture systems
Owing to the complexity of terpene biosynthetic pathways, a single chassis cell often fails to provide optimal expression of all key enzymes. Effective expression of these enzymes is crucial for maintaining flux balance, minimizing metabolic burden, and enhancing catalytic efficiency. Introducing a coculture system divides the biosynthetic pathway into independent catalytic modules, with each module hosted by an appropriate cell type [142]. This approach addresses challenges related to complexity (Fig. 2H), offering several advantages: (1) minimizing interference between pathway modules; (2) reducing the metabolic load on each host cell; (3) allowing precise optimization of each module by adjusting strain ratios; and (4) enabling flexible, plug-and-play biosynthesis of diverse products. Qi et al. used E. coli to synthesize β-ionone but observed excessive accumulation of dihydro-β-ionone without complete conversion [143]. To resolve this, they introduced a coculture system, where E. coli produced β-ionone and S. cerevisiae expressing the DBR1 module converted it to dihydro-β-ionone. Optimizing strain ratios elevated the yield to 27 mg/L, representing a 3.4-fold increase compared with using a single strain. Tang et al. developed a modular coculture system for sclareolide production using S. cerevisiae and Cryptococcus neoformans ATCC 20918 [144]. First, a recombinant S. cerevisiae strain was engineered to produce 536.2 mg/L of sclareol. Subsequently, Hyphozyma roseonigra was used to catalyze its conversion to sclareolide. By optimizing coculture conditions, the final yield reached 626.3 mg/L, emphasizing the potential of coculture strategies for sustainable sclareolide biosynthesis.
Fermentation optimization strategies
In the industrialization of microbial synthesis for terpenoid fragrances, bioreactor fermentation is an important method for evaluating the physiological characteristics and economic productivity of microbial strains. However, during the scale-up process, variations in environmental parameters, such as increased shear stress and formation of dissolved oxygen gradients, can result in the so-called “scale effect,” which disrupts metabolic flux and reduces product synthesis efficiency [145, 146]. To mitigate these adverse effects, strain adaptation strategies have proven effective. For example, Huang et al. examined the synthesis of α-ionone using E. coli and found that a significant amount of H₂O₂ was generated during large-scale cultivation. This reactive species caused oxidative degradation of lycopene, a key precursor, thereby reducing the overall yield. To overcome this problem, they overexpressed alkyl hydroperoxide reductase AhpC/F in the engineered strain to efficiently remove excess H₂O₂, thereby restoring lycopene accumulation. This resulted in a yield of 700 mg/L of α-ionone in a 5-L bioreactor, demonstrating enhanced oxidative stress tolerance and improved stability for large-scale production [147].
The two-stage fermentation strategy has been widely adopted in fermentation. By separating the microbial growth phase from the product synthesis phase, this approach facilitates the efficient biosynthesis of terpenoid flavor compounds. The fundamental principle of this strategy involves isolating biomass accumulation, which predominantly occurs during the exponential growth phase under favorable nutritional and environmental conditions, from targeted product formation, which occurs during the stationary phase through interventions, such as carbon source switching, temperature or pH modulation, and activation of inducible promoters within the metabolic pathway. By alleviating the constraints imposed by growth-associated carbon flux allocation and redirecting metabolic flux toward the desired biosynthetic route, this method significantly enhances the spatiotemporal productivity and process robustness [148]. Zhang et al. used dynamic regulation of key genes in the sterol biosynthesis pathway using a glucose-responsive promoter to establish an effective two-stage fermentation system [88]. During the glucose-rich phase, competing pathways were suppressed to prioritize cell proliferation. Upon glucose depletion, these inhibitory effects were relieved automatically, thereby initiating β-caryophyllene biosynthesis. In fed-batch fermentation, an initial glucose-containing medium was used to enhance biomass generation, followed by a switch to an ethanol-based medium combined with in situ extraction techniques, which markedly improved the efficiency of metabolic flux redirection. The resulting yield of β-caryophyllene increased by 10.5-fold compared with that of the original strain, reaching 21.4 g/L. This demonstrates the substantial benefits of the two-stage fermentation strategy for terpenoid compound synthesis.
Optimizing the fermentation process through precise dynamic regulation of key environmental parameters, such as pH, dissolved oxygen, and temperature, as well as strategies such as two-phase cultivation can alleviate external stress and provide a more favorable growth environment for micro-organisms. This facilitates stable or even high-yield production of terpenoid compounds [149]. For example, Shukal et al. demonstrated that microaerobic fermentation can serve as an effective strategy to overcome yield limitations by modulating redox homeostasis and metabolic flux distribution. The underlying mechanism involves the significant inhibition of geraniol oxidative degradation into citronellal under low-oxygen conditions, which also reduces the metabolic burden. By replacing traditional dissolved oxygen control with redox potential regulation in a 5-L fermenter and precisely maintaining a low-oxygen reducing state through the dynamic adjustment of aeration and agitation rates, the geranyl acetate yield was increased to 19 g/L [30]. Furthermore, because of the highly hydrophobic nature of most terpenoid flavor compounds, the application of two-phase fermentation for in situ extraction not only enhances product stability but also significantly boosts synthesis efficiency. In a study of trans-nerolidol synthesis using E. coli by Tan et al., the implementation of a dodecane-based two-phase extraction system markedly improved large-scale production performance. In particular, the organic phase efficiently captured 94% of the hydrophobic products from the aqueous phase while reducing gas-phase volatilization loss by over 3.4%. This enabled in situ product removal, alleviated intracellular accumulation inhibition, and increased the yield from 7 g/L in single-phase fermentation to 16 g/L within 93 h in a 5-L bioreactor, resulting in an improvement of 128% [47].
In conclusion, strategies for regulating the microbial environment and optimizing the fermentation process are important for overcoming the “scale effect.” By implementing approaches, such as microaerobic fermentation, two-phase extraction, and two-stage cultivation, the synthesis efficiency and process robustness of terpenoids may be significantly enhanced.
Outlook
Although significant progress has been made in the production of certain terpenoids, such as geraniol and limonene, through biosynthetic approaches, current production costs for these compounds remain higher than those incurred using conventional chemical synthesis methods. For many other high-value terpenoids (e.g., ambrein), biosynthesis yields and cost efficiency are below the thresholds required for commercial viability. Therefore, substantially enhancing biosynthetic efficiency to achieve economically competitive production levels is required for the future large-scale industrial adoption of microbial terpenoid biosynthesis. A major challenge is the low catalytic activity of terpene synthases, necessitating a deeper understanding of their native expression patterns and environmental conditions to optimize heterologous expression and fermentation processes. High-density fermentation is often required to achieve high terpenoid yields. However, many terpenoids exhibit cytotoxicity, and their excessive accumulation during fermentation can inhibit microbial cell growth. Consequently, strategies such as separating the growth and production phases, enhancing host tolerance through adaptive evolution or genetic engineering, and leveraging unconventional hosts with higher solvent or pH tolerance could improve production. Nevertheless, gene editing tools for many unconventional hosts remain inefficient, highlighting the need for advanced genome engineering technologies. With the rapid advancements in synthetic biology and metabolic engineering, microbial cell factories have emerged as a highly promising platform for terpenoid biosynthesis, offering the potential to enable more scalable, sustainable, and cost-effective industrial production in the future.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Author contributions
Q.L. writing—original draft, visualization, resources, investigation, data curation, and conceptualization; Y.N. writing—original draft, visualization, data curation; D.L., J.R., and C.L. resources and data curation; Z.C. writing—review and editing, supervision, project administration, and conceptualization; G.W. writing—review and editing, supervision, project administration, funding acquisition, and conceptualization. All authors reviewed the manuscript.
Funding
This work was supported by the Technical Program of China Tobacco Henan Industrial Co., Ltd., (AW2024005).
Data availability
No data sets were generated or analyzed during the current study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhifei Chen, Email: chenzhifei@126.com.
Guanglu Wang, Email: wangguanglu@zzuli.edu.cn.
References
- 1.Chandran SS, Kealey JT, Reeves CD. Microbial production of isoprenoids. Process Biochem. 2011;46(9):1703–10. 10.1016/j.procbio.2011.05.012. [Google Scholar]
- 2.Moser S, Pichler H. Identifying and engineering the ideal microbial terpenoid production host. Appl Microbiol Biotechnol. 2019;103(14):5501–16. 10.1007/s00253-019-09892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masyita A, Sari RM, Astuti AD, Yasir B, Rumata NR, Emran TB, et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem: X. 2022;30(13):100217. 10.1016/j.fochx.2022.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vickers CE, Williams TC, Peng B, Cherry J. Recent advances in synthetic biology for engineering isoprenoid production in yeast. Curr Opin Chem Biol. 2017;40:47–56. 10.1016/j.cbpa.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Shang J, Feng D, Liu H, Niu L, Li R, Li Y, et al. Evolution of the biosynthetic pathways of terpene scent compounds in roses. Curr Biol. 2024;34(15):3550–63. 10.1016/j.cub.2024.06.075. [DOI] [PubMed] [Google Scholar]
- 6.Bell EL, Finnigan W, France SP, Green AP, Hayes MA, Hepworth LJ, et al. Biocatalysis. Nat Rev Methods Primers. 2021;1(1):46. 10.1038/s43586-021-00044-z. [Google Scholar]
- 7.Schneider A, Lystbaek TB, Markthaler D, Hansen N, Hauer B. Biocatalytic stereocontrolled head-to-tail cyclizations of unbiased terpenes as a tool in chemoenzymatic synthesis. Nat Commun. 2024;15(1):4925. 10.1038/s41467-024-48993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Zhang C, Lindley ND. Metabolic engineering strategies for sustainable terpenoid flavor and fragrance synthesis. J Agric Food Chem. 2020;68(38):10252–64. 10.1021/acs.jafc.9b06203. [DOI] [PubMed] [Google Scholar]
- 9.Schempp FM, Drummond L, Buchhaupt M, Schrader J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J Agric Food Chem. 2018;66(10):2247–58. 10.1021/acs.jafc.7b00473. [DOI] [PubMed] [Google Scholar]
- 10.Meadows AL, Hawkins KM, Tsegaye Y, Antipov E, Kim Y, Raetz L, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature. 2016;537(7622):694–7. 10.1038/nature19769. [DOI] [PubMed] [Google Scholar]
- 11.Wu W, Maravelias CT. Synthesis and techno-economic assessment of microbial-based processes for terpenes production. Biotechnol Biofuels. 2018;11:294. 10.1186/s13068-018-1285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun C, Theodoropoulos C, Scrutton NS. Techno-economic assessment of microbial limonene production. Bioresour Technol. 2020;300:122666. 10.1016/j.biortech.2019.122666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamabe Y, Kawagoe Y, Okuno K, Inoue M, Chikaoka K, Ueda D, et al. Construction of an artificial system for ambrein biosynthesis and investigation of some biological activities of ambrein. Sci Rep. 2020;10(1):19643. 10.1038/s41598-020-76624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeung AWK, Tzvetkov NT, Gupta VK, Gupta SC, Orive G, Bonn GK, et al. Current research in biotechnology: exploring the biotech forefront. Curr Res Biotechnol. 2019;1:34–40. 10.1016/j.crbiot.2019.08.003. [Google Scholar]
- 15.Kim J, Salvador M, Saunders E, Gonzalez J, Avignone-Rossa C, Jimenez JI. Properties of alternative microbial hosts used in synthetic biology: towards the design of a modular chassis. Essays Biochem. 2016;60(4):303–13. 10.1042/EBC20160015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Liang H, Gao R, Qin L, Xu P, Huang M, et al. Yeast metabolism adaptation for efficient terpenoids synthesis via isopentenol utilization. Nat Commun. 2024;15(1):9844. 10.1038/s41467-024-54298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao Y, Liu M, Fordjour E, Yu P, Yang Y, Liu X, et al. Engineering Escherichia coli for perillyl alcohol production with reduced endogenous dehydrogenation. ACS Synth Biol. 2025. 10.1021/acssynbio.4c00854. [DOI] [PubMed] [Google Scholar]
- 18.Cha YP, Li W, Wu T, You X, Chen HF, Zhu CY, et al. Probing the synergistic ratio of P450/CPR to improve (+)-nootkatone production in Saccharomyces cerevisiae. J Agric Food Chem. 2022;70(3):815–25. 10.1021/acs.jafc.1c07035. [DOI] [PubMed] [Google Scholar]
- 19.Cheng YT, Luo LL, Tang H, Wang J, Ren L, Cui GH, et al. Engineering the microenvironment of P450s to enhance the production of diterpenoids in Saccharomyces cerevisiae. Acta Pharm Sin B. 2024;14(10):4608–18. 10.1016/j.apsb.2024.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava G, Vyas P, Kumar A, Singh A, Bhargav P, Dinday S, et al. Unraveling the role of cytochrome P450 enzymes in oleanane triterpenoid biosynthesis in arjuna tree. Plant J. 2024;119(6):2687–705. 10.1111/tpj.16942. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Zu Y, Huang S, Stephanopoulos G. Engineering a universal and efficient platform for terpenoid synthesis in yeast. Proc Natl Acad Sci U S A. 2023;120(1):e2207680120. 10.1073/pnas.2207680120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganjave SD, Dodia H, Sunder AV, Madhu S, Wangikar PP. High cell density cultivation of E. coli in shake flasks for the production of recombinant proteins. Biotechnol Rep. 2022;33:e00694. 10.1016/j.btre.2021.e00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng Y, Jiang T, Yan Y. The expanded CRISPR toolbox for constructing microbial cell factories. Trends Biotechnol. 2024;42(1):104–18. 10.1016/j.tibtech.2023.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanijou JK, Hee YT, Scipion CPM, Chen X, Selvarajoo K. Systems biology approach for enhancing limonene yield by re-engineering Escherichia coli. NPJ Syst Biol Appl. 2024;10(1):109. 10.1038/s41540-024-00440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster LA, Reisch CR. Plasmids for controlled and tunable high level expression in E coli. Appl Enviro Microbiol. 2022;88(22):e0093922. 10.1128/aem.00939-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zong Z, Hua Q, Tong X, Li D, Wang C, Guo D, et al. Biosynthesis of nerol from glucose in the metabolic engineered Escherichia coli. Bioresour Technol. 2019;287:121410. 10.1016/j.biortech.2019.121410. [DOI] [PubMed] [Google Scholar]
- 27.Lei D, Qiu Z, Wu J, Qiao B, Qiao J, Zhao GR. Combining metabolic and monoterpene synthase engineering for de Novo production of monoterpene alcohols in Escherichia coli. ACS Synth Biol. 2021;10(6):1531–44. 10.1021/acssynbio.1c00081. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Xu X, Zhang R, Cheng T, Cao Y, Li X, et al. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol Biofuels. 2016;9:58. 10.1186/s13068-016-0466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Chen J, Zhang J, Zhou Y, Zhang Y, Wang F, et al. Engineering Escherichia coli for production of geraniol by systematic synthetic biology approaches and laboratory-evolved fusion tags. Metab Eng. 2021;66:60–7. 10.1016/j.ymben.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Shukal S, Ong L, T R, Chen X, Zhang C. Microaerobic fermentation enables high-titer biosynthesis of the rose monoterpenes geraniol and geranyl acetate in Escherichia coli. ACS Sustain Chem Eng. 2024;12(10):3921–32. 10.1021/acssuschemeng.3c06030. [Google Scholar]
- 31.Wu J, Cheng S, Cao J, Qiao J, Zhao GR. Systematic optimization of limonene production in engineered Escherichia coli. J Agric Food Chem. 2019;67(25):7087–97. 10.1021/acs.jafc.9b01427. [DOI] [PubMed] [Google Scholar]
- 32.Willrodt C, David C, Cornelissen S, BüHLER B, Julsing MK, Schmid A. Engineering the productivity of recombinant Escherichia coli for limonene formation from glycerol in minimal media. Biotechnol J. 2014;9(8):1000–12. 10.1002/biot.201400023. [DOI] [PubMed] [Google Scholar]
- 33.Rolf J, Julsing MK, Rosenthal K, Lutz S. A gram-scale limonene production process with engineered Escherichia coli. Mol. 2020. 10.3390/molecules25081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weston-Green K, Clunas H, Jimenez Naranjo C. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: discovering novel therapeutics in the flavours and fragrances of cannabis. Front Psychiatry. 2021;12:583211. 10.3389/fpsyt.2021.583211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickey RM, Gopal MR, Nain P, Kunjapur AM. Recent developments in enzymatic and microbial biosynthesis of flavor and fragrance molecules. J Biotechnol. 2024;389:43–60. 10.1016/j.jbiotec.2024.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Bokinsky G, Peralta-Yahya PP, George A, Holmes BM, Steen EJ, Dietrich J, et al. Synthesis of three advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia coli. Proc Natl Acad Sci U S A. 2011;108(50):19949–54. 10.1073/pnas.1106958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Nie Q, Ren M, Feng H, Jiang X, Zheng Y, et al. Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol Biofuels. 2013;6(1):60. 10.1186/1754-6834-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarria S, Wong B, Martín HG, Keasling JD, Peralta-Yahya P. Microbial synthesis of pinene. ACS Synth Biol. 2014;3(7):466–75. 10.1021/sb4001382. [DOI] [PubMed] [Google Scholar]
- 39.Huang MY, Wang WY, Liang ZZ, Huang YC, Yi Y, Niu FX. Enhancing the production of pinene in Escherichia coli by using a combination of shotgun, product-tolerance and I-SceI cleavage systems. Biology. 2022;11(10):1484. 10.3390/biology11101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao SH, Zhang DY, Meng E. Improving biosynthetic production of pinene through plasmid recombination elimination and pathway optimization. Plasmid. 2019;105:102431. 10.1016/j.plasmid.2019.102431. [DOI] [PubMed] [Google Scholar]
- 41.Wei LJ, Zhong YT, Nie MY, Liu SC, Hua Q. Biosynthesis of alpha-Pinene by genetically engineered Yarrowia lipolytica from low-cost renewable feedstocks. J Agric Food Chem. 2021;69(1):275–85. 10.1021/acs.jafc.0c06504. [DOI] [PubMed] [Google Scholar]
- 42.Niu FX, Huang YB, Shen YP, Ji LN, Liu JZ. Enhanced production of pinene by using a cell-free system with modular cocatalysis. J Agric Food Chem. 2020;68(7):2139–45. 10.1021/acs.jafc.9b07830. [DOI] [PubMed] [Google Scholar]
- 43.Dunlop MJ, Dossani ZY, Szmidt HL, Chu HC, Lee TS, Keasling JD, et al. Engineering microbial biofuel tolerance and export using efflux pumps. Mol Syst Biol. 2011;7(1):487. 10.1038/msb.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su P, Hu T, Liu Y, Tong Y, Guan H, Zhang Y, et al. Functional characterization of NES and GES responsible for the biosynthesis of (E)-nerolidol and (E,E)-geranyllinalool in Tripterygium wilfordii. Sci Rep. 2017;7:40851. 10.1038/srep40851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng B, Plan MR, Chrysanthopoulos P, Hodson MP, Nielsen LK, Vickers CE. A squalene synthase protein degradation method for improved sesquiterpene production in Saccharomyces cerevisiae. Metab Eng. 2017;39:209–19. 10.1016/j.ymben.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Li W, Zhang W, Liu Z, Song H, Wang S, Zhang Y, et al. Review of recent advances in microbial production and applications of nerolidol. J Agric Food Chem. 2025;73(10):5724–47. 10.1021/acs.jafc.4c12579. [DOI] [PubMed] [Google Scholar]
- 47.Tan N, Ong L, Shukal S, Chen X, Zhang C. High-yield biosynthesis of trans-nerolidol from sugar and glycerol. J Agric Food Chem. 2023;71(22):8479–87. 10.1021/acs.jafc.3c01161. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Park JE, Choi ES, Kim SW. Farnesol production in Escherichia coli through the construction of a farnesol biosynthesis pathway - application of PgpB and YbjG phosphatases. Biotechnol J. 2016;11(10):1291–7. 10.1002/biot.201600250. [DOI] [PubMed] [Google Scholar]
- 49.Kong S, Fu X, Li X, Pan H, Guo D. De novo biosynthesis of linalool from glucose in engineered Escherichia coli. Enzyme Microb Technol. 2020;140:109614. 10.1016/j.enzmictec.2020.109614. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, Wang X, Xiao L, Wang F, Zhang Y, Li X. Synthetic protein scaffolds for improving R-(-)-linalool production in Escherichia coli. J Agric Food Chem. 2021;69(20):5663–70. 10.1021/acs.jafc.1c01101. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Zhang X, Zhang J, Xiao L, Zhou Y, Wang F, et al. Metabolic engineering of Escherichia coli for efficient production of linalool from biodiesel-derived glycerol by targeting cofactors regeneration and reducing acetate accumulation. Ind Crops Prod. 2023;203:117152. 10.1016/j.indcrop.2023.117152. [Google Scholar]
- 52.Li X, Ren JN, Fan G, Zhang LL, Pan SY. Advances on (+)-nootkatone microbial biosynthesis and its related enzymes. J Ind Microbiol Biotechnol. 2021. 10.1093/jimb/kuab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Girhard M, Machida K, Itoh M, Schmid RD, Arisawa A, Urlacher VB. Regioselective biooxidation of (+)-valencene by recombinant E. coli expressing CYP109B1 from Bacillus subtilis in a two-liquid-phase system. Microb Cell Fact. 2009;8(1):36. 10.1186/1475-2859-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang J, Wei X, Liu D, Li Q, Li C, Zhao J, et al. Engineering Escherichia coli via introduction of the isopentenol utilization pathway to effectively produce geranyllinalool. Microb Cell Fact. 2024;23(1):292. 10.1186/s12934-024-02563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schalk M, Pastore L, Mirata MA, Khim S, Schouwey M, Deguerry F, et al. Toward a biosynthetic route to sclareol and amber odorants. J Am Chem Soc. 2012;134(46):18900–3. 10.1021/ja307404u. [DOI] [PubMed] [Google Scholar]
- 56.Cheng T, Zhao G, Xian M, Xie C. Improved cis-Abienol production through increasing precursor supply in Escherichia coli. Sci Rep. 2020;10(1):16791. 10.1038/s41598-020-73934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, Wang X, Li X, Shi H, Wang F, Zhang Y, et al. Combinatorial engineering of mevalonate pathway and diterpenoid synthases in Escherichia coli for cis-abienol production. J Agric Food Chem. 2019;67(23):6523–31. 10.1021/acs.jafc.9b02156. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Zhu K, Shi H, Wang X, Zhang Y, Wang F, et al. Engineering Escherichia coli for effective and economic production of cis-abienol by optimizing isopentenol utilization pathway. J Clean Prod. 2022;351:131310. 10.1016/j.jclepro.2022.131310. [Google Scholar]
- 59.Yang H, Zhang K, Shen W, Chen L, Xia Y, Zou W, et al. Efficient production of cembratriene-ol in Escherichia coli via systematic optimization. Microb Cell Fact. 2023;22(1):17. 10.1186/s12934-023-02022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schrepfer P, Ugur I, Klumpe S, Loll B, Kaila VRI, Bruck T. Exploring the catalytic cascade of cembranoid biosynthesis by combination of genetic engineering and molecular simulations. Comput Struct Biotechnol J. 2020;18:1819–29. 10.1016/j.csbj.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang G, Wei X, Li Q, Chang J, Yang X. Metabolic engineering of Escherichia coli for enhanced production of cembratrien-ols via precursor supply optimization and membrane engineering. J Agric Food Chem. 2025. 10.1021/acs.jafc.5c01254. [DOI] [PubMed] [Google Scholar]
- 62.Ke D, Caiyin Q, Zhao F, Liu T, Lu W. Heterologous biosynthesis of triterpenoid ambrein in engineered Escherichia coli. Biotechnol Lett. 2018;40(2):399–404. 10.1007/s10529-017-2483-2. [DOI] [PubMed] [Google Scholar]
- 63.Naseri G. A roadmap to establish a comprehensive platform for sustainable manufacturing of natural products in yeast. Nat Commun. 2023;14(1):1916. 10.1038/s41467-023-37627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee H, Song J, Seo SW. Engineering Yarrowia lipolytica for the production of beta-carotene by carbon and redox rebalancing. J Biol Eng. 2025;19(1):6. 10.1186/s13036-025-00476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou K, Yu C, Liang N, Xiao W, Wang Y, Yao M, et al. Adaptive evolution and metabolic engineering boost lycopene production in Saccharomyces cerevisiae via enhanced precursors supply and utilization. J Agric Food Chem. 2023;71(8):3821–31. 10.1021/acs.jafc.2c08579. [DOI] [PubMed] [Google Scholar]
- 66.Bureau JA, Oliva ME, Dong Y, Ignea C. Engineering yeast for the production of plant terpenoids using synthetic biology approaches. Nat Prod Rep. 2023;40(12):1822–48. 10.1039/d3np00005b. [DOI] [PubMed] [Google Scholar]
- 67.Zhou P, Du Y, Xu N, Yue C, Ye L. Improved linalool production in Saccharomyces cerevisiae by combining directed evolution of linalool synthase and overexpression of the complete mevalonate pathway. Biochem Eng J. 2020;161:107655. 10.1016/j.bej.2020.107655. [Google Scholar]
- 68.Zhou P, Du Y, Fang X, Xu N, Yue C, Ye L. Combinatorial modulation of linalool synthase and farnesyl diphosphate synthase for linalool overproduction in Saccharomyces cerevisiae. J Agric Food Chem. 2021;69(3):1003–10. 10.1021/acs.jafc.0c06384. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Cao X, Wang J, Tang F. Enhancement of linalool production in Saccharomyces cerevisiae by utilizing isopentenol utilization pathway. Microb Cell Fact. 2022;21(1):212. 10.1186/s12934-022-01934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang C, Li M, Zhao GR, Lu W. Alpha-terpineol production from an engineered Saccharomyces cerevisiae cell factory. Microb Cell Fact. 2019;18(1):160. 10.1186/s12934-019-1211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J, Bao X, Li C, Shen Y, Hou J. Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2016;100(10):4561–71. 10.1007/s00253-016-7375-1. [DOI] [PubMed] [Google Scholar]
- 72.Zhao C, Wang XH, Lu XY, Zong H, Zhuge B. Tuning geraniol biosynthesis via a novel decane-responsive promoter in Candida glycerinogenes. ACS Synth Biol. 2022;11(5):1835–44. 10.1021/acssynbio.2c00003. [DOI] [PubMed] [Google Scholar]
- 73.Zhao C, Wang XH, Lu XY, Zong H, Zhuge B. Metabolic engineering of Candida glycerinogenes for sustainable production of geraniol. ACS Synth Biol. 2023;12(6):1836–44. 10.1021/acssynbio.3c00195. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Daviet L, Schalk M, Siewers V, Nielsen J. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metab Eng. 2013;15:48–54. 10.1016/j.ymben.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Scalcinati G, Knuf C, Partow S, Chen Y, Maury J, Schalk M, et al. Dynamic control of gene expression in engineered for the production of plant sesquitepene α-santalene in a fed-batch mode. Metab Eng. 2012;14(2):91–103. 10.1016/j.ymben.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Zha W, An T, Li T, Zhu J, Gao K, Sun Z, et al. Reconstruction of the biosynthetic pathway of santalols under control of the GAL regulatory system in yeast. ACS Synth Biol. 2020;9(2):449–56. 10.1021/acssynbio.9b00479. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Wang X, Zhang X, Zhang Y, Wang F, Li X. Sesquiterpene synthase engineering and targeted engineering of alpha-santalene overproduction in Escherichia coli. J Agric Food Chem. 2022;70(17):5377–85. 10.1021/acs.jafc.2c00754. [DOI] [PubMed] [Google Scholar]
- 78.Qu Z, Zhang L, Zhu S, Yuan W, Hang J, Yin D, et al. Overexpression of the transcription factor HAC1 improves nerolidol production in engineered yeast. Enzyme Microb Technol. 2020;134:109485. 10.1016/j.enzmictec.2019.109485. [DOI] [PubMed] [Google Scholar]
- 79.Li W, Yan X, Zhang Y, Liang D, Caiyin Q, Qiao J. Characterization of trans-Nerolidol synthase from Celastrus angulatus Maxim and production of trans-Nerolidol in engineered Saccharomyces cerevisiae. J Agric Food Chem. 2021;69(7):2236–44. 10.1021/acs.jafc.0c06084. [DOI] [PubMed] [Google Scholar]
- 80.Sabulal B, Dan M, J AJ, Kurup R, Pradeep NS, Valsamma RK, et al. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: chemical characterization and antimicrobial activity. Phytochemistry. 2006;67(22):2469–73. 10.1016/j.phytochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Styger G, Prior B, Bauer FF. Wine flavor and aroma. J Ind Microbiol Biotechnol. 2011;38(9):1145–59. 10.1007/s10295-011-1018-4. [DOI] [PubMed] [Google Scholar]
- 82.Harvey BG, Meylemans HA, Gough RV, Quintana RL, Garrison MD, Bruno TJ. High-density biosynthetic fuels: the intersection of heterogeneous catalysis and metabolic engineering. Phys Chem Chem Phys. 2014;16(20):9448–57. 10.1039/c3cp55349c. [DOI] [PubMed] [Google Scholar]
- 83.Lu S, Deng H, Zhou C, Du Z, Guo X, Cheng Y, et al. Enhancement of beta-caryophyllene biosynthesis in Saccharomyces cerevisiae via synergistic evolution of beta-caryophyllene synthase and engineering the chassis. ACS Synth Biol. 2023;12(6):1696–707. 10.1021/acssynbio.3c00024. [DOI] [PubMed] [Google Scholar]
- 84.Cheng T, Zhang K, Guo J, Yang Q, Li Y, Xian M, et al. Highly efficient biosynthesis of beta-caryophyllene with a new sesquiterpene synthase from tobacco. Biotechnol Biofuels Bioprod. 2022;15(1):39. 10.1186/s13068-022-02136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang J, Li Z, Guo L, Du J, Bae H-J. Biosynthesis of β-caryophyllene, a novel terpene-based high-density biofuel precursor, using engineered Escherichia coli. Renew Energy. 2016;99:216–23. 10.1016/j.renene.2016.06.061. [Google Scholar]
- 86.Gietz RD, Schiestl RH. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2(1):31–4. 10.1038/nprot.2007.13. [DOI] [PubMed] [Google Scholar]
- 87.Li Z, Gan Y, Gou C, Ye Q, Wu Y, Wu Y, et al. Efficient biosynthesis of beta-caryophyllene in Saccharomyces cerevisiae by beta-caryophyllene synthase from Artemisia argyi. Synth Syst Biotechnol. 2025;10(1):158–64. 10.1016/j.synbio.2024.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Liu C, Li W, Ma Z, Lv B, Qin L, et al. Systematic engineering of the sterol synthesis pathway for Saccharomyces cerevisiae promotes the efficient production of β-caryophyllene. Metab Eng. 2025;91:347–55. 10.1016/j.ymben.2025.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Wang J, Zhu L, Li Y, Xu S, Jiang W, Liang C, et al. Enhancing geranylgeraniol production by metabolic engineering and utilization of isoprenol as a substrate in Saccharomyces cerevisiae. J Agric Food Chem. 2021;69(15):4480–9. 10.1021/acs.jafc.1c00508. [DOI] [PubMed] [Google Scholar]
- 90.Wang K, Yin M, Sun ML, Zhao Q, Ledesma-Amaro R, Ji XJ, et al. Engineering Yarrowia lipolytica for efficient synthesis of geranylgeraniol. J Agric Food Chem. 2024;72(37):20568–81. 10.1021/acs.jafc.4c06749. [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Li Y, Jiang W, Hu J, Gu Z, Xu S, et al. Engineering Saccharomyces cerevisiae YPH499 for overproduction of geranylgeraniol. J Agric Food Chem. 2023;71(25):9804–14. 10.1021/acs.jafc.3c01820. [DOI] [PubMed] [Google Scholar]
- 92.Ouyang X, Cha Y, Li W, Zhu C, Zhu M, Li S, et al. Stepwise engineering of Saccharomyces cerevisiae to produce (+)-valencene and its related sesquiterpenes. RSC Adv. 2019;9(52):30171–81. 10.1039/c9ra05558d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu T, Li W, Chen H, Wu T, Zhu C, Zhuo M, et al. Systematic optimization of HPO-CPR to boost (+)-nootkatone synthesis in engineered Saccharomyces cerevisiae. J Agric Food Chem. 2022;70(49):15548–59. 10.1021/acs.jafc.2c07068. [DOI] [PubMed] [Google Scholar]
- 94.Sun M-L, Han Y, Yu X, Wang K, Lin L, Ledesma-Amaro R, et al. Constructing a green oleaginous yeast cell factory for sustainable production of the plant-derived diterpenoid sclareol. Green Chem. 2024;26(9):5202–10. 10.1039/D3GC04949C. [Google Scholar]
- 95.Cao X, Yu W, Chen Y, Yang S, Zhao ZK, Nielsen J, et al. Engineering yeast for high-level production of diterpenoid sclareol. Metab Eng. 2023;75:19–28. 10.1016/j.ymben.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 96.Moser S, Strohmeier GA, Leitner E, Plocek TJ, Vanhessche K, Pichler H. Whole-cell (+)-ambrein production in the yeast Pichia pastoris. Metab Eng Commun. 2018;7:e00077. 10.1016/j.mec.2018.e00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin C, Zhang X, Ji Z, Fan B, Chen Y, Wu Y, et al. Metabolic engineering of Saccharomyces cerevisiae for high-level production of (+)-ambrein from glucose. Biotechnol Lett. 2024;46(4):615–26. 10.1007/s10529-024-03502-2. [DOI] [PubMed] [Google Scholar]
- 98.Kapoor L, Ramamoorthy S. Strategies to meet the global demand for natural food colorant bixin: a multidisciplinary approach. J Biotechnol. 2021;338:40–51. 10.1016/j.jbiotec.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 99.Debnath T, Bandyopadhyay TK, Vanitha K, Bobby MN, Nath Tiwari O, Bhunia B, et al. Astaxanthin from microalgae: a review on structure, biosynthesis, production strategies and application. Food Res Int. 2024;176:113841. 10.1016/j.foodres.2023.113841. [DOI] [PubMed] [Google Scholar]
- 100.Cao K, Cui Y, Sun F, Zhang H, Fan J, Ge B, et al. Metabolic engineering and synthetic biology strategies for producing high-value natural pigments in microalgae. Biotechnol Adv. 2023;68:108236. 10.1016/j.biotechadv.2023.108236. [DOI] [PubMed] [Google Scholar]
- 101.Cataldo VF, Lopez J, Carcamo M, Agosin E. Chemical vs. biotechnological synthesis of C13-apocarotenoids: current methods, applications and perspectives. Appl Microbiol Biotechnol. 2016;100(13):5703–18. 10.1007/s00253-016-7583-8. [DOI] [PubMed] [Google Scholar]
- 102.Lin P, Zhang L, Du G, Chen J, Zhang J, Peng Z. Construction of Saccharomyces cerevisiae platform strain for the biosynthesis of carotenoids and apocarotenoids. J Agric Food Chem. 2025;73(15):9187–96. 10.1021/acs.jafc.5c00088. [DOI] [PubMed] [Google Scholar]
- 103.Ma Y, Liu N, Greisen P, Li J, Qiao K, Huang S, et al. Removal of lycopene substrate inhibition enables high carotenoid productivity in Yarrowia lipolytica. Nat Commun. 2022;13(1):572. 10.1038/s41467-022-28277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bian Q, Zhou P, Yao Z, Li M, Yu H, Ye L. Heterologous biosynthesis of lutein in S. cerevisiae enabled by temporospatial pathway control. Metab Eng. 2021;67:19–28. 10.1016/j.ymben.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 105.Kim GB, Kim HR, Lee SY. Comprehensive evaluation of the capacities of microbial cell factories. Nat Commun. 2025;16(1):2869. 10.1038/s41467-025-58227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng B, Wei S. Synthetic engineering of microbes for production of terpenoid food ingredients. J Agric Food Chem. 2025. 10.1021/acs.jafc.5c01724. [DOI] [PubMed] [Google Scholar]
- 107.Han T, Nazarbekov A, Zou X, Lee SY. Recent advances in systems metabolic engineering. Curr Opin Biotechnol. 2023;84:103004. 10.1016/j.copbio.2023.103004. [DOI] [PubMed] [Google Scholar]
- 108.Tan JC, Hu Q, Scrutton NS. A growth-coupling strategy for improving the stability of terpenoid bioproduction in Escherichia coli. Microb Cell Fact. 2024;23(1):279. 10.1186/s12934-024-02548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diaz JE, Lin CS, Kunishiro K, Feld BK, Avrantinis SK, Bronson J, et al. Computational design and selections for an engineered, thermostable terpene synthase. Protein Sci. 2011;20(9):1597–606. 10.1002/pro.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woolston BM, Edgar S, Stephanopoulos G. Metabolic engineering: past and future. Annu Rev Chem Biomol Eng. 2013;4(1):259–88. 10.1146/annurev-chembioeng-061312-103312. [DOI] [PubMed] [Google Scholar]
- 111.Zhang C, Chen X, Lindley ND, Too HP. A “plug-n-play” modular metabolic system for the production of apocarotenoids. Biotechnol Bioeng. 2018;115(1):174–83. 10.1002/bit.26462. [DOI] [PubMed] [Google Scholar]
- 112.Lu Y, Yang Q, Lin Z, Yang X. A modular pathway engineering strategy for the high-level production of beta-ionone in Yarrowia lipolytica. Microb Cell Fact. 2020;19(1):49. 10.1186/s12934-020-01309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eauclaire SF, Zhang J, Rivera CG, Huang LL. Combinatorial metabolic pathway assembly in the yeast genome with RNA-guided Cas9. J Ind Microbiol Biotechnol. 2016;43(7):1001–15. 10.1007/s10295-016-1776-0. [DOI] [PubMed] [Google Scholar]
- 114.Lian J, Hamedirad M, Hu S, Zhao H. Combinatorial metabolic engineering using an orthogonal tri-functional CRISPR system. Nat Commun. 2017;8(1):1688. 10.1038/s41467-017-01695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang XK, Wang DN, Chen J, Liu ZJ, Wei LJ, Hua Q. Metabolic engineering of β-carotene biosynthesis in Yarrowia lipolytica. Biotechnol Lett. 2020;42(6):945–56. 10.1007/s10529-020-02844-x. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y, Ma L, Su P, Huang L, Gao W. Cytochrome P450s in plant terpenoid biosynthesis: discovery, characterization and metabolic engineering. Crit Rev Biotechnol. 2023;43(1):1–21. 10.1080/07388551.2021.2003292. [DOI] [PubMed] [Google Scholar]
- 117.Bilal M, Iqbal HMN. Tailoring multipurpose biocatalysts via protein engineering approaches: a review. Catal Lett. 2019;149(8):2204–17. 10.1007/s10562-019-02821-8. [Google Scholar]
- 118.Park SY, Eun H, Lee MH, Lee SY. Metabolic engineering of Escherichia coli with electron channelling for the production of natural products. Nat Catal. 2022;5(8):726–37. 10.1038/s41929-022-00820-4. [Google Scholar]
- 119.Kang W, Ma X, Kakarla D, Zhang H, Fang Y, Chen B, et al. Organizing enzymes on self-assembled protein cages for cascade reactions. Angew Chem Int Ed Engl. 2022;61(52):e202214001. 10.1002/anie.202214001. [DOI] [PubMed] [Google Scholar]
- 120.Wu T, Ye L, Zhao D, Li S, Li Q, Zhang B, et al. Membrane engineering - a novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli. Metab Eng. 2017;43(Pt A):85–91. 10.1016/j.ymben.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 121.Sun ZJ, Lian JZ, Zhu L, Jiang YQ, Li GS, Xue HL, et al. Combined biosynthetic pathway engineering and storage pool expansion for high-level production of ergosterol in industrial Saccharomyces cerevisiae. Front Bioeng Biotechnol. 2021;9:681666. 10.3389/fbioe.2021.681666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo XJ, Yao MD, Xiao WH, Wang Y, Zhao GR, Yuan YJ. Compartmentalized reconstitution of post-squalene pathway for 7-dehydrocholesterol overproduction in Saccharomyces cerevisiae. Front Microbiol. 2021;12:663973. 10.3389/fmicb.2021.663973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi Y, Wang D, Li R, Huang L, Dai Z, Zhang X. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides. Metab Eng. 2021;67:104–11. 10.1016/j.ymben.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 124.Wang D, Wang J, Shi Y, Li R, Fan F, Huang Y, et al. Elucidation of the complete biosynthetic pathway of the main triterpene glycosylation products of Panax notoginseng using a synthetic biology platform. Metab Eng. 2020;61:131–40. 10.1016/j.ymben.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 125.Choi BH, Kang HJ, Kim SC, Lee PC. Organelle engineering in yeast: enhanced production of Protopanaxadiol through manipulation of peroxisome proliferation in Saccharomyces cerevisiae. Microorganisms. 2022. 10.3390/microorganisms10030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Du MM, Zhu ZT, Zhang GG, Zhao YQ, Gao B, Tao XY, et al. Engineering Saccharomyces cerevisiae for hyperproduction of beta-amyrin by mitigating the inhibition effect of squalene on beta-amyrin synthase. J Agric Food Chem. 2022;70(1):229–37. 10.1021/acs.jafc.1c06712. [DOI] [PubMed] [Google Scholar]
- 127.Dong C, Shi Z, Huang L, Zhao H, Xu Z, Lian J. Cloning and characterization of a panel of mitochondrial targeting sequences for compartmentalization engineering in Saccharomyces cerevisiae. Biotechnol Bioeng. 2021;118(11):4269–77. 10.1002/bit.27896. [DOI] [PubMed] [Google Scholar]
- 128.Yao Z, Zhou P, Su B, Su S, Ye L, Yu H. Enhanced isoprene production by reconstruction of metabolic balance between strengthened precursor supply and improved isoprene synthase in Saccharomyces cerevisiae. ACS Synth Biol. 2018;7(9):2308–16. 10.1021/acssynbio.8b00289. [DOI] [PubMed] [Google Scholar]
- 129.Fordjour E, Mensah EO, Hao Y, Yang Y, Liu X, Li Y, et al. Toward improved terpenoids biosynthesis: strategies to enhance the capabilities of cell factories. Bioresour Bioprocess. 2022;9(1):6. 10.1186/s40643-022-00493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bu X, Lin JY, Cheng J, Yang D, Duan CQ, Koffas M, et al. Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of beta-carotene in Saccharomyces cerevisiae. Biotechnol Biofuels. 2020;13:168. 10.1186/s13068-020-01809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu T, Li S, Ye L, Zhao D, Fan F, Li Q, et al. Engineering an artificial membrane vesicle trafficking system (AMVTS) for the excretion of beta-carotene in Escherichia coli. ACS Synth Biol. 2019;8(5):1037–46. 10.1021/acssynbio.8b00472. [DOI] [PubMed] [Google Scholar]
- 132.Matsumoto T, Osawa T, Taniguchi H, Saito A, Yamada R, Ogino H. Mitochondrial expression of metabolic enzymes for improving carotenoid production in Saccharomyces cerevisiae. Biochem Eng J. 2022;189:108720. 10.1016/j.bej.2022.108720. [Google Scholar]
- 133.Dusseaux S, Wajn WT, Liu Y, Ignea C, Kampranis SC. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc Natl Acad Sci U S A. 2020;117(50):31789–99. 10.1073/pnas.2013968117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baker JJ, Shi J, Wang S, Mujica EM, Bianco S, Capponi S, et al. ML-enhanced peroxisome capacity enables compartmentalization of multienzyme pathway. Nat Chem Biol. 2024. 10.1038/s41589-024-01759-2. [DOI] [PubMed] [Google Scholar]
- 135.Niu FX, He X, Wu YQ, Liu JZ. Enhancing production of pinene in Escherichia coli by using a combination of tolerance, evolution, and modular co-culture engineering. Front Microbiol. 2018;9:1623. 10.3389/fmicb.2018.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu C, You X, Wu T, Li W, Chen H, Cha Y, et al. Efficient utilization of carbon to produce aromatic valencene in Saccharomyces cerevisiae using mannitol as the substrate. Green Chem. 2022;24(11):4614–27. 10.1039/d2gc00867j. [Google Scholar]
- 137.Li J, Zhu K, Miao L, Rong L, Zhao Y, Li S, et al. Simultaneous improvement of limonene production and tolerance in Yarrowia lipolytica through tolerance engineering and evolutionary engineering. ACS Synth Biol. 2021;10(4):884–96. 10.1021/acssynbio.1c00052. [DOI] [PubMed] [Google Scholar]
- 138.Karlova R, Busscher J, Schempp FM, Buchhaupt M, VAN Dijk ADJ, Beekwilder J. Detoxification of monoterpenes by a family of plant glycosyltransferases. Phytochem. 2022. 10.1016/j.phytochem.2022.113371. [DOI] [PubMed] [Google Scholar]
- 139.Rinaldi MA, Ferraz CA, Scrutton NS. Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli. Nat Prod Rep. 2022;39(1):90–118. 10.1039/d1np00025j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Priebe X, Hoang MD, Rudiger J, Turgel M, Trondle J, Schwab W, et al. Byproduct-free geraniol glycosylation by whole-cell biotransformation with recombinant Escherichia coli. Biotechnol Lett. 2021;43(1):247–59. 10.1007/s10529-020-02993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang X, Zhang X, Zhang J, Xiao L, Zhou Y, Zhang Y, et al. Genetic and bioprocess engineering for the selective and high-level production of geranyl acetate in Escherichia coli. ACS Sustain Chem Eng. 2022;10(9):2881–9. 10.1021/acssuschemeng.1c07336. [Google Scholar]
- 142.He N, Li D-F, Yu H-W, Ye L-D. Construction of an artificial microbial consortium for green production of (−)-ambroxide. ACS Sustain Chem Eng. 2023;11(5):1939–48. 10.1021/acssuschemeng.2c06716. [Google Scholar]
- 143.Qi Z, Tong X, Ke K, Wang X, Pei J, Bu S, et al. De novo synthesis of dihydro-beta-ionone through metabolic engineering and bacterium-yeast coculture. J Agric Food Chem. 2024;72(6):3066–76. 10.1021/acs.jafc.3c07291. [DOI] [PubMed] [Google Scholar]
- 144.Tang D, Zheng X, Zhao Y, Zhang C, Chen C, Chen Y, et al. Engineered microbial consortium for de novo production of Sclareolide. J Agric Food Chem. 2024;72(36):19977–84. 10.1021/acs.jafc.4c05506. [DOI] [PubMed] [Google Scholar]
- 145.Nadal-Rey G, Mcclure DD, Kavanagh JM, Cornelissen S, Fletcher DF, Gernaey KV. Understanding gradients in industrial bioreactors. Biotechnol Adv. 2021;46:107660. 10.1016/j.biotechadv.2020.107660. [DOI] [PubMed] [Google Scholar]
- 146.Kuschel M, Takors R. Simulated oxygen and glucose gradients as a prerequisite for predicting industrial scale performance a priori. Biotechnol Bioeng. 2020;117(9):2760–70. 10.1002/bit.27457. [DOI] [PubMed] [Google Scholar]
- 147.Huang CN, Lim X, Ong L, Lim C, Chen X, Zhang C. Mediating oxidative stress enhances alpha-ionone biosynthesis and strain robustness during process scaling up. Microb Cell Fact. 2022;21(1):246. 10.1186/s12934-022-01968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen M, Li M, Ye L, Yu H. Construction of canthaxanthin-producing yeast by combining spatiotemporal regulation and pleiotropic drug resistance engineering. ACS Synth Biol. 2022;11(1):325–33. 10.1021/acssynbio.1c00437. [DOI] [PubMed] [Google Scholar]
- 149.Kang MK, Yoon SH, Kwon M, Kim SW. Microbial cell factories for bio-based isoprenoid production to replace fossil resources. Curr Opin Syst Biol. 2024. 10.1016/j.coisb.2023.100502. [Google Scholar]
- 150.Sun C, Zhang R, Xie C. Biosynthesis of (R)-(+)-perillyl alcohol by Escherichia coli expressing neryl pyrophosphate synthase. Eng Life Sci. 2022;22(5):407–16. 10.1002/elsc.202100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang X, Wang J, Zhang X, Zhang J, Zhou Y, Wang F, et al. Efficient myrcene production using linalool dehydratase isomerase and rational biochemical process in Escherichia coli. J Biotechnol. 2023;371:33–40. 10.1016/j.jbiotec.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 152.Lim HS, Kim SK, Woo SG, Kim TH, Yeom SJ, Yong W, et al. (-)-alpha-bisabolol production in engineered Escherichia coli expressing a novel (-)-alpha-bisabolol synthase from the globe artichoke cynara cardunculus var scolymus. J Agric Food Chem. 2021;69(30):8492–503. 10.1021/acs.jafc.1c02759. [DOI] [PubMed] [Google Scholar]
- 153.Sun Y, Wu S, Fu X, Lai C, Guo D. De novo biosynthesis of tau-cadinol in engineered Escherichia coli. Bioresour Bioprocess. 2022;9(1):29. 10.1186/s40643-022-00521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fordjour E, Liu CL, Hao Y, Sackey I, Yang Y, Liu X, et al. Engineering Escherichia coli BL21 (DE3) for high‐yield production of germacrene A, a precursor of β‐elemene via combinatorial metabolic engineering strategies. Biotechnol Bioeng. 2023;120(10):3039–56. 10.1002/bit.28467. [DOI] [PubMed] [Google Scholar]
- 155.Zhou L, Wang Y, Han L, Wang Q, Liu H, Cheng P, et al. Enhancement of patchoulol production in Escherichia coli via multiple engineering strategies. J Agric Food Chem. 2021;69(27):7572–80. 10.1021/acs.jafc.1c02399. [DOI] [PubMed] [Google Scholar]
- 156.Zhang H, Cai P, Guo J, Gao J, Xie L, Su P, et al. Engineering cellular dephosphorylation boosts (+)-borneol production in yeast. Acta Pharm Sin B. 2025;15(2):1171–82. 10.1016/j.apsb.2024.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang G, Yao M, Wang Y, Xiao W, Yuan Y. A “push-pull-restrain” strategy to improve citronellol production in Saccharomyces cerevisiae. Metab Eng. 2021;66:51–9. 10.1016/j.ymben.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 158.Liu J, Chen C, Wan X, Yao G, Bao S, Wang F, et al. Identification of the sesquiterpene synthase AcTPS1 and high production of (-)-germacrene D in metabolically engineered Saccharomyces cerevisiae. Microb Cell Fact. 2022;21(1):89. 10.1186/s12934-022-01814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu M, Lin YC, Guo JJ, Du MM, Tao X, Gao B, et al. High-level production of sesquiterpene patchoulol in Saccharomyces cerevisiae. ACS Synth Biol. 2021;10(1):158–72. 10.1021/acssynbio.0c00521. [DOI] [PubMed] [Google Scholar]
- 160.Feng P, Sun B, Bi H, Bao Y, Wang M, Zhang H, et al. Developing thermosensitive metabolic regulation strategies in the fermentation process of Saccharomyces cerevisiae to enhance alpha-bisabolene production. ACS Synth Biol. 2025;14(4):1129–41. 10.1021/acssynbio.4c00728. [DOI] [PubMed] [Google Scholar]
- 161.Zhang Y, Bian S, Liu X, Fang N, Wang C, Liu Y, et al. Synthesis of cembratriene-ol and cembratriene-diol in yeast via the MVA pathway. Microb Cell Fact. 2021;20(1):29. 10.1186/s12934-021-01523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data sets were generated or analyzed during the current study.




