Abstract
The proximity of humans to small mammals and their ectoparasites at wildlife-human interfaces in Africa creates favorable conditions for the spillover of zoonotic pathogens. Anaplasmaceae and Rickettsiaceae bacteria are emerging threats due to their genomic adaptability and resilience to environmental changes. This systematic review and meta-analysis examined the distribution and prevalence of these pathogens in African small mammals and their ectoparasites. A comprehensive search across five electronic databases yielded 37 studies meeting predefined inclusion and exclusion criteria. The most frequently reported pathogens were Rickettsia felis (11 studies), Rickettsia massiliae (8 studies), and Rickettsia typhi (7 studies). A meta-analysis using a random-effects model was conducted separately for small mammals and ectoparasites. In small mammals, the highest prevalence was observed for Anaplasma spp. (15.17%; 95% CI 8-26.9%), Rickettsia helvetica (14.65%; 95% CI 9.09–22.75%), R. felis (12%; 95% CI 6.13–22.18%), R. massiliae (10.75%; 95% CI 0.6-70.55%), and Ehrlichia spp. (10.35%; 95% CI 4.13–23.66%). Among ectoparasites, the highest prevalence was found in ticks for R. africae (41.53%; 95% CI 13.53–76.33%) and R. massiliae (30.55%; 95% CI 8.96–66.29%). In fleas, R. felis (21.68%; 95% CI 8.67–44.68%) was most prevalent. This analysis underscores the importance of molecular detection tools, such as the combination of quantitative real-time polymerase chain reaction (qPCR) and sequencing for detection and identification of Rickettsiales, and highlights the dominance of R. africae, R. felis, and R. massiliae in ectoparasites, particularly in Eastern and Northern Africa. The study also underscores the impact of sample size and geographical regional differences in prevalence estimates. This review was registered in the international database of Prospectively Registered Systematic Reviews (PROSPERO) with ID: CRD42024552324.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-11434-z.
Keywords: Small mammals, Ectoparasites, Anaplasmaceae, Rickettsiaceae, Rickettsiales, Africa
Introduction
The emergence and re-emergence of zoonotic diseases present major challenges to both public and veterinary health in Africa. These challenges are exacerbated by limited resources, underdeveloped health infrastructure, and a high burden of infectious diseases. Zoonoses contribute significantly to human morbidity and mortality and reduce livestock productivity, particularly in poor and marginalized communities where misdiagnosis and underreporting are common [1, 2]. Globally, over 60% of emerging pathogens in humans are zoonotic, with approximately 72% originating from wildlife [3, 4]. It is estimated that more than 2.5 billion cases of zoonotic infections occur each year worldwide [1]. Bacterial pathogens especially those in the order Rickettsiales, account for over 54% of reported emerging infectious diseases globally, many of which are associated with antimicrobial resistance [3].
In Africa, zoonotic infections contribute to more than 25% of the disease burden. This is closely linked to poverty, frequent human-animal interactions (particularly with livestock and wildlife), and immunosuppression in vulnerable populations [5]. The threat of zoonotic transmission is expected to rise due to several interrelated biological, ecological, environmental, and demographic drivers. These include rapid population growth, increased human mobility and trade, armed conflict, environmental degradation, climate change, and shifting human behaviors [6, 7]. Such factors intensify contact between humans, wildlife, and domestic animals, increasing opportunities for pathogen spillover and cross-species transmission. These conditions facilitate the emergence and spread of zoonotic diseases.
Many of these interactions occur at the human-animal interface and involve small mammals such as rodents and shrews, which live in close proximity to human settlements [8–11]. Their ectoparasites also thrive in these environments, further increasing the risk of zoonotic spillover [5, 12]. Understanding these complex interactions is essential, particularly in relation to vector-borne diseases. Non-volant small mammals including rodents, shrews, hedgehogs, and small carnivores, act as reservoirs for a wide array of zoonotic pathogens. Their adaptability to human habitats and widespread presence across African ecosystems make them key players in disease ecology [3, 13]. Historically, rodents were central to the spread of diseases like the bubonic plague, highlighting their importance in zoonotic transmission [14].
Small mammals frequently harbor diverse ectoparasites, such as ticks, fleas, mites, and lice. These ectoparasites can act as vectors or amplification hosts for pathogens, including Rickettsiales bacteria. The distribution and prevalence of these parasites are influenced by host population dynamics and environmental conditions such as climate, weather variability, and land-use change [15]. Numerous studies have documented the association of specific ectoparasites with different small mammal hosts across Africa [16–18]. As human activities increasingly encroach upon natural habitats, the likelihood of zoonotic spillover events rises, heightening public health risks.
Bacteria belonging to the families Rickettsiaceae and Anaplasmataceae, associated with small mammals and their ectoparasites, pose a potentially significant but underexplored threat to public and veterinary health in Africa [19]. These are obligate intracellular pathogens, including genera such as Rickettsia, Anaplasma, Ehrlichia, and Orientia. Rickettsia species are broadly categorized into three groups: the Spotted Fever Group (SFG; e.g., R. rickettsii, R. conorii, R. africae), the Typhus Group (TG; e.g., R. prowazekii, R. typhi), and the Ancestral Group (AG; e.g., R. bellii, R. canadensis). These bacteria are associated with specific arthropod vectors and geographical distributions, contributing to their global epidemiological significance.
Rickettsiales pathogens cause a variety of diseases in humans and animals and rely on both invertebrate vectors and small mammals for transmission [20]. In Africa’s diverse ecosystems, the zoonotic spread of Rickettsiaceae and Anaplasmataceae is a growing concern. For instance, Anaplasma phagocytophilum, the causative agent of human granulocytic anaplasmosis, has been identified in rodents [21, 22]. Several zoonotic Rickettsia species including R. africae, R. conorii, R. helvetica (SFG), and R. typhi (TG), have also been reported in small mammals [23, 24]. Among these, R. africae, which causes African tick-bite fever (ATBF), is the most frequently reported in Africa and is the second most common cause of febrile illness in travelers returning from sub-Saharan Africa [25, 26].
In response to the rising burden of zoonoses, multiple studies have explored the ecological and epidemiological dynamics at animal-human interfaces in Africa and beyond. However, most of these studies are fragmented and country-specific, limiting our understanding of the broader distribution and prevalence of Rickettsiaceae and Anaplasmataceae across the continent. Moreover, no comprehensive synthesis has evaluated the roles of various small mammal species and their ectoparasites as potential transmission routes for these pathogens.
This systematic review and meta-analysis aim to consolidate fragmented epidemiological data on zoonotic Anaplasmataceae and Rickettsiaceae pathogens associated with small mammals and their ectoparasites in Africa. The specifically we aim to: (1) identify and describe the main groups of small mammals and ectoparasites associated with these bacterial agents in Africa; (2) map the geographic distribution of reported Rickettsiaceae and Anaplasmataceae species across African countries; (3) use meta-analysis to estimate pooled prevalence of selected Rickettsiales agents among different groups of small mammals and ectoparasites; (4) evaluate the statistical significance of moderators affecting prevalence estimates through subgroup analysis and meta-regression.
The motivation for conducting this study stems from the scarcity of reliable data on the prevalence and impact of Rickettsiales pathogens in small mammals and ectoparasites in Africa. This is despite their anticipated importance in zoonotic disease transmission. Many African communities face elevated risk due to close interactions with livestock and wildlife, exacerbated by poverty, inadequate infrastructure, and limited access to healthcare. Increasing agricultural expansion and livestock rearing near protected areas has led to significant encroachment into wildlife habitats, creating conditions favorable for pathogen spillover and cross-species transmission. This review therefore provides a comprehensive overview of the ecological and epidemiological roles of small mammals and their ectoparasites in the transmission of Rickettsiales. The findings will serve as a valuable reference for researchers, public health authorities, and policymakers involved in zoonotic disease surveillance and control in Africa.
Materials and methods
Focus area of study
This systematic review considered only original field-evidenced publications reported on potential zoonotic Rickettsiales pathogens associated with small mammals and their ectoparasites in Africa reported between the year 2000 to 2024 inclusive.
Search strategy
This systematic review and meta-analysis was registered in the international database of Prospectively Registered Systematic Reviews (PROSPERO) with the following ID: CRD42024552324, can be accessed from PROSPERO website (https://www.crd.york.ac.uk/PROSPERO/home). The review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [27]. We applied the Population–Intervention–Comparison–Outcome (PICO) guide model [28] to develop focused research questions and define the inclusion and exclusion criteria for selecting studies in this review. This structured approach allowed for a systematic assessment of the literature. In this context, the population (P) of interest comprised small mammals and their ectoparasites found in Africa. The intervention (I) referred to the use of molecular or serological diagnostic methods to detect Rickettsiales bacteria. The comparison (C) involved evaluating differences among test results within or between studied groups. The outcome (O) was defined as the presence or absence of Rickettsiales bacteria, including Rickettsia, Anaplasma, and Ehrlichia species. Thus, based on the PICO framework, we formulated four key hypothetical research questions to guide this review: (1) Which populations of small mammals or their respective ectoparasites in Africa are associated with Rickettsia, Anaplasma, or Ehrlichia species, and what is the estimated prevalence of these bacteria? (2) Which molecular or serological diagnostic methods are most commonly used to detect Rickettsiales agents in small mammals and/or their ectoparasites in Africa, and how effective are these methods? (3) Which circulating Anaplasmataceae and Rickettsiaceae species identified in African small mammals and/or ectoparasites are known or suspected to be potentially zoonotic? (4) What is the potential epidemiological role of the studied small mammal and ectoparasite populations in the transmission of Rickettsiales pathogens to humans in Africa?
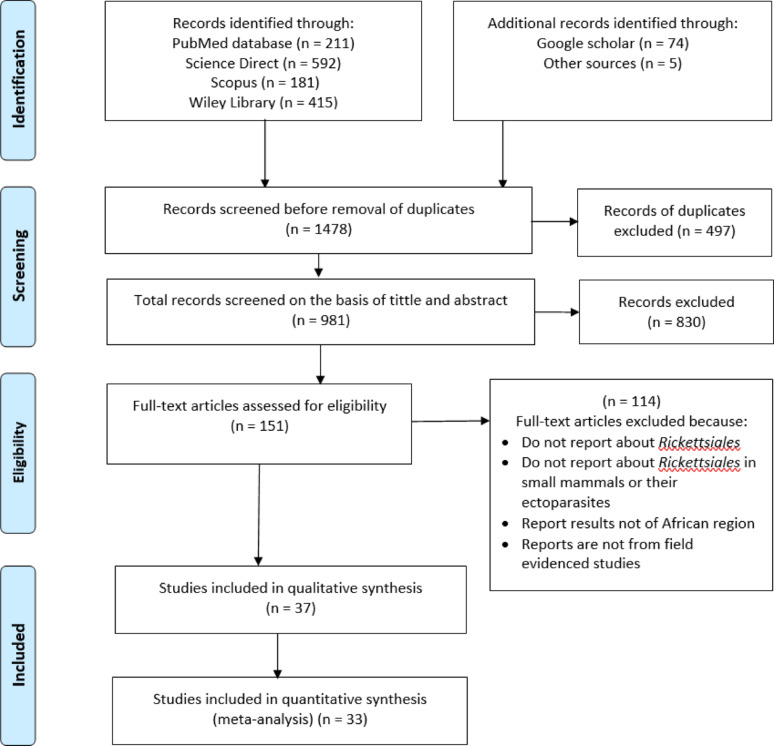
The literature search was performed through various indexed databases including PubMed, Science Direct, SCOPUS and Wiley Library. It also included additional relevant articles from other search such as Google Scholar and articles referencing follow-up. Only articles published in the English language between the years 2000 to 2024 inclusive were retrieved and included for this review (Fig. 1). To maximise the search and reduce selection bias, the key search terms “syntax” included were (“small mammals” OR non-volant OR “non volant” OR mammal OR rodent OR rat OR jird OR gerbil OR vole OR mouse OR hamster OR porcupines OR hedgehog OR squirrel OR jerboa OR shrew) AND (Rickettsiales OR anaplasma OR anaplasmosis OR ehrlichia OR ehrlichiosis OR rickettsia OR rickettsiosis OR ricketts* OR “spotted fever” OR neoehrlichia OR orientia OR Wolbachia OR “vector borne” OR “tick borne” ) AND (Ectoparasite OR flea OR mites OR lice OR tick) AND (55 individual Africa country names). In advanced screening for search, the following criteria were used: [Title/Abstract] in PubMed, [Topic] in Science Direct, [TITLE-ABS-KEY] in Scopus, and [anywhere] in Wiley Library. The search process was repeated just before the final analysis process to ensure the capturing of the most recent studies eligible to be included in our review study. Zotero (v. 6.0.36) was used to process the data searched and also it was used to identify and remove the duplicate data searched.
Fig. 1.
Systematic search and selection of articles included for this review
Selection strategies
Inclusion and exclusion criteria
All articles retrieved through searching were initially screened by checking the tittle and abstract, and then investigate the full-text to ensure eligibility of the article to be included in this review. Article is considered eligible only if (i) it was original field evidenced report describing information related to occurrence, diagnostics or diversity of Anaplasmaceae or Rickettsiaceae infecting small mammals and/or their respective ectoparasites; (ii) it was reported from either of the 55 African countries as defined by African Union (AU) [29]; (iii) it was published in English language between the year 2000 and 2024 inclusive.
Altenatively, an article was reasonably excluded from this review following one or more of the following exclusion criteria: (i) interested populations are humans, companion animals, livestock or higher wildlife; and ectoparasites explicitly not stated as associated with small mammals; (ii) the study is for therapeutic or treatment laboratory control tests, including dosage tests; (iii) case control studies or in vitro experimental control studies; (iv) targeted pathogen or microbial agent investigated is different from Anaplasmaceae and Rickettsiaceae; (v) the study is a review article, conference abstract, symposia, poster session or oral presentation, published before the year 2000 or in 2025; (vi) the study conducted beyond African countries.
Data extraction and critical assessment of included studies
Data were extracted from eligible articles and filled in the prepared Excel sheet templates for seven groups of variables from full-text retrieved: (i) publication specifics (including authors’ names, study year, publication year, country and region of study in Africa); (ii) animal studied specifics (group of animal such as rodents, shrews, hedgehogs etc. with their species); (iii) ectoparasites specifics (groups of ectoparasites such as ticks, fleas, lice and mites with their species); (iv) infestation specifics (particular group of ectoparasite(s) infesting a particular group of small mammal(s); (v) laboratory test specifics (such as conventional PCR (cPCR), qualitative real-time PCR (qPCR) or Enzyme linked immunosorbent assay (ELISA) test alone or accompanied with sequencing or multi locus sequence typing (MLST)); (vi) epidemiological specifics (qualitative or quantitative data for Rickettsiales detections reported); (vii) pathogen specifics (detected pathogen species of Rickettsia, Anaplasma or Ehrlichia).
For reliability of reviewal process and data extraction: Two members of the team; one from Sokoine university of Agriculture working together independently from one of University of Dar es Salaam were directly involved in data search and selection; the third member of the team for Tanzania ministry of Education, Science and Technology was working as convener to compare the results from two independent members. In the event of any discrepancies or inconsistencies in the data between two independent team members, the entire team convened to discuss the issue and reach a consensus on the appropriate course of action.
Risk of bias
This review has certain limitations, particularly concerning missing publications and publication bias. The search strategy was designed to retrieve as many relevant publications as possible while also refining the results to focus on studies most pertinent to this review. However, it is highly likely that relevant research articles lacking the specified keywords in their titles or abstracts were inadvertently overlooked. Additionally, as all selected publications were obtained through electronic searches, we acknowledge a potential bias toward studies published online.
Most of the included publications employed a cross-sectional design, reporting on the detection of Rickettsiaceae and Anaplasmaceae pathogens in small mammals and/or their associated ectoparasites at a specific point in time. Cross-sectional studies are inherently susceptible to selection and information bias. In addition, another source of bias for such studies may stem from selecting sampled organisms or animals based solely on their availability. We utilized a modified version of the Appraisal Tool for Cross-Sectional Studies (AXIS) for critical evaluation of the risk of bias for individual included cross-sectional studies [30]. This tool comprises 20 checklist-based questions with response options of “yes,” “no,” or “don’t know.” However, because our review focused exclusively on small mammals and their ectoparasites, then, questions 7, 13, and 14 which were designed for human subjects, were deemed inapplicable. For studies below 50% positive score were interpreted as “high” risk of bias, 50–70% positive score as “moderate” and those exceeding 70% were considered to have a low risk of bias.
Data analysis
The manually transcribed data was stored in a Microsoft Excel spreadsheet. R studio (version 2024.04.1–748) programming software was used for qualitative and quantitative manipulation and statistical analysis of data. Summary of qualitative analysis was described in tables, bar plots and maps.
We conducted a meta-analysis to quantitatively estimate the pooled molecular prevalence of Rickettsiales infections and the intensity of ectoparasites infestation. This analysis included only outcome measures reported in at least 10 datasets across different studies. The pooled prevalence estimate served as the measure of effect size in the meta-analysis. Results were presented using forest plots and further summarized in tables for clarity. For subgroup analysis, a minimum of five datasets per subgroup (moderator) was required for inclusion. This ensured statistical robustness in the comparison across groups.
To assess variability between study datasets, we adopted a random-effects model rather than a fixed-effects model. This choice was based on the assumption that significant heterogeneity existed in the prevalence estimates of Rickettsiales across different small mammal and ectoparasite populations, leading to differences in the true effect size across studies. Heterogeneity was evaluated using chi-square (X2) test under Cochran’s Q statistics considering the p-Value, and Higgins and Thompson’s I² statistic, along with prediction intervals (PI) to estimate the expected range of future prevalence values based on current evidence [31]. The I2 statistic describes the percentage of variability in the effect measure that is due to variation between studies rather than the sampling error [31]. The I2 value is defined as: Low: ≤ 25%, Moderate: 25–50%, Substantial: 50–75% and Considerable: ≥ 75%.
To explore potential sources of heterogeneity, we performed subgroup analysis and meta-regression, assuming that different subgroups (moderators) each with its overall effect to the general pooled effect size which may be the underlying source of heterogeneity (variability) among studies. The following seven moderators were predefined in advance: sample source (type of animal vis. rodents/shrews/hedgehogs etc. or ectoparasite vis. tick/flea/louse/mite); detection method (molecular or serological test); sampling country; geographic region (sub-region within Africa); sampling period (categorical time periods); sample size; host genus (animal or ectoparasite genus). Subgroup analysis was conducted only when heterogeneity was substantial, defined as I² >70%.
To assess potential publication bias, we applied the small-study effects method, following the approach by Egger et al. [32]. This method is based on the assumption that smaller studies reporting high prevalence rates are more likely to be published. Publication bias was evaluated by inspecting funnel plot asymmetry, both visually and statistically using Egger’s regression test. A p-value < 0.05 in Egger’s test was considered indicative of significant asymmetry. When bias was detected, the Duval and Tweedie Trim-and-Fill method was used to adjust for missing studies, employing the L0 estimator to impute these missing values [33].
For multiple meta-regression analysis, we employed a stepwise regression approach to identify significant predictors among the moderators. Moderators that were statistically significant in subgroup analysis were initially included. Then, based on model fit indices—residual I² and R²—non-significant moderators were removed iteratively until the optimal model fit was achieved.
Results
Qualitative analysis
Studies description
A total of 37 studies published between 2005 and 2024 were retrieved and included in the qualitative analysis. Of these, 33 studies met the criteria for inclusion in the meta-analysis (Fig. 1). These studies reported on the occurrence, detection, and identification of potential zoonotic bacteria from the order Rickettsiales in small mammals and/or their associated ectoparasites specifically ticks, fleas, mites, and lice (see Supplementary Table S1). All included articles were field evidenced study, though they varied in study design, sampling methods, identification techniques, and sample sizes.
Most studies (30/37; 81%) included for this review were conducted within last decade, the period from 2014 to 2024 (Fig. 2). The period demonstrates a considerable increase of research interest in surveillances of zoonotic Anaplasmaceae and Rickettsiaceae from small mammals and/or their associated ectoparasites along animal-human interfaces.
Fig. 2.
Number of studies reported on the detection of Rickettsiales bacteria under families Rickettsiaceae and Anaplasmaceae from small mammals and/or their associated ectoparasites, published from 2005 to 2024
Ectoparasites infestation in small mammals’ studies related to Rickettsiales
A total of 31 genera belonging to four groups of small mammals—rodents, shrews, hedgehogs, and mongooses—were recorded. Rodents accounted for the majority, representing 22 out of 31 genera (71%), reported in 21 studies. Shrews contributed (3/31; 9.7%), found in 5 studies, while mongooses also accounted for (3/31; 9.7%), reported in 2 studies. Hedgehogs and squirrels each contributed 1 genus (3.2%), with hedgehogs recorded in 3 studies and squirrels in one.
Among rodents, Rattus spp. was the most frequently reported genus, appearing in 49 of 90 datasets (54.4%) across 11 studies—mostly represented by R. rattus. This was followed by Mastomys spp. (19/90; 21.1%), mainly M. natalensis, in 7 studies, and Mus spp. (15/90; 16.7%), mostly M. musculus, in 6 studies. Although Rhabdomys spp. comprised 11.1% (10/90) of records, it was reported in only one study from South Africa.
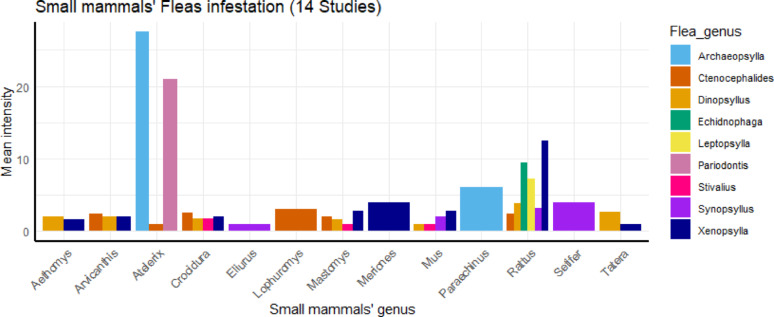
Fleas were the most commonly reported ectoparasite group associated with Rickettsiales infections, appearing in 70 out of 117 datasets from 14 studies in Africa. Nine flea genera were identified. Xenopsylla spp., particularly the oriental rat flea X. cheopis, was the most frequent, associated with eight different small mammal groups. Ctenocephalides spp., especially the cat flea C. felis, was the second most reported genus, found on five host groups.
Rattus spp. was the most frequently infested host, carrying five flea genera in high abundance. This was followed by Mus, Mastomys, and Crocidura spp., each infested by four flea genera (Fig. 3).
Fig. 3.
Distribution of flea groups infesting particular genus of small mammals in studies related to Rickettsiales in Africa
Six tick genera were reported in association with Rickettsiales infections in small mammals across Africa, appearing in 38 of 117 datasets from 10 studies. Rhipicephalus spp. was the most frequently reported, found on 11 small mammal groups, with R. sanguineus being the most common species. Haemaphysalis spp. was the second most frequently reported genus, associated with 8 host groups, followed by Ixodes spp., which was recorded in 4 host groups.
Hedgehogs of the genus Atelerix were the most heavily infested hosts. They were parasitized by five tick genera: Amblyomma, Haemaphysalis, Hyalomma, Ixodes, and Rhipicephalus. The rodent Thryonomys was the next most infested, reported with three tick genera (Fig. 4).
Fig. 4.
Distribution of tick groups infesting particular genus of small mammals in studies related to Rickettsiales in Africa
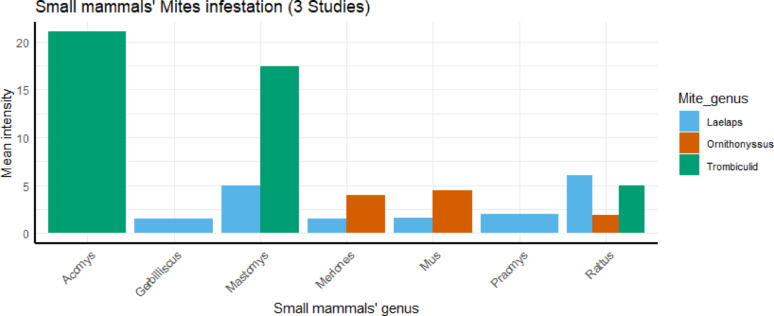
However, only 3 groups of mites—Laelaps spp., Ornithonyssus spp., and Trombiculid mites infesting small mammals appearing in 14 out of 117 datasets reported from 3 studies (Fig. 5). In addition, only one genus of lice—Hoplopleura spp., (2/117 in 2 studies) was reported to infest rodent specifically R. rattus associated with Rickettsiales infection in Africa.
Fig. 5.
Distribution of mite groups infesting particular genus of small mammals in studies related to Rickettsiales in Africa
Geographical distribution of Rickettsiales infecting small mammals in Africa
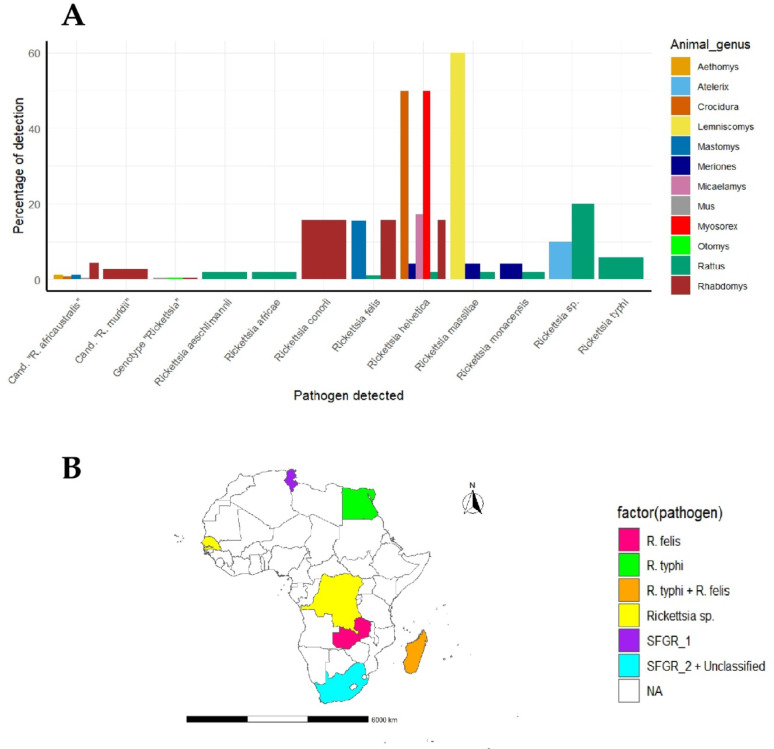
Among the eligible articles included in this review, bacteria from the Rickettsiaceae family, particularly the genus Rickettsia, were the most frequently reported—appearing in 30 out of 37 studies (81%) (see Supplementary Table S1). Among small mammals, rodents were the most commonly reported hosts of Rickettsia infections, with 35 out of 39 datasets from 8 studies. This was followed by shrews (3 datasets from 1 study) and hedgehogs (1 dataset from 1 study). Rattus spp. was the most frequently reported rodent host, appearing in 11 of the 39 datasets, based on studies from Tunisia, Egypt, Madagascar, the Democratic Republic of the Congo (DRC), and Senegal. Rhabdomys spp. was the second most common, reported in 10 datasets, but all from a single study in South Africa. Mastomys spp. was recorded in two datasets from studies in Zambia and South Africa.
In total, eight Rickettsia species were reported to infect small mammals in Africa. Seven belonged to the Spotted Fever Group (SFG): R. aeschlimannii, R. africae, R. conorii, R. felis, R. helvetica, R. massiliae, and R. monacensis. These were reported in 18 datasets from four studies conducted in South Africa, Zambia, Madagascar and Tunisia. Only one Typhus Group (TGR) species, R. typhi, was identified, found in 2 datasets from Madagascar and Egypt.
Unclassified Rickettsia (including Candidatus Rickettsia species and unnamed genotypes) accounted for 15 datasets, all from a single study in South Africa. In addition, Rickettsia sp. was reported in 4 datasets from studies in DRC, Senegal, and Tunisia. The distribution of Rickettsia species across different small mammal genera in Africa is illustrated in Fig. 6A and B.
Fig. 6.
Distribution of Rickettsia species infecting various genus of small mammals in Africa: A Percentage of animals detected with particular Rickettsia pathogen, B Geographical distribution of animal detected with a particular Rickettsia pathogen in Africa. Cand. “R = Candidatus “Rickettsia; SFGR_1 = Rickettsia helvetica + R. monacensis + R. aeschlimannii + R. massiliae + Rickettsia sp.; SFGR_2 + Unclassified = Rickettsia conorii + R. felis + R. helvetica + R. massiliae + Cand. “R. africaustralis” + Cand.“R. muridii” + Genotype “Rickettsia”
Rodents were the most frequently reported hosts of Anaplasma species, appearing in 15 out of 17 datasets from 5 studies. In contrast, mongooses and shrews were each reported only once, in a single study. Rattus spp. was the only genus reported in three datasets from two studies conducted in Tunisia and Gabon. Other small mammal species were reported only once across the remaining datasets. Two identified Anaplasma species were found in small mammals in Africa. A. phagocytophilum was detected in 8 datasets from 2 studies in Tunisia and Kenya. A. ovis was reported in Rattus spp. in a single study from Tunisia. Additionally, an uncharacterized strain, Candidatus “A. gabonensis”, was identified in one study from Gabon.
Two studies from Senegal reported Anaplasma spp. in 3 datasets, infecting various small mammals.
Ehrlichia species were also reported in small mammals, primarily in rodents (6 out of 9 datasets from 4 studies), and in hedgehogs (3 datasets from 1 study). E. ewingii was the only identified species infecting hedgehogs (Atelerix spp.) in Tunisia. Candidatus “E. senegalensis” and Candidatus “E. shimanensis” were detected in rodents and hedgehogs from Senegal and Tunisia, respectively. The distribution of Anaplasma and Ehrlichia species across small mammal genera in Africa is shown in Fig. 7A and B.
Fig. 7.
Distribution of Anaplasmaceae species infecting various genus of small mammals in Africa: A Percentage of animals detected with particular Anapasmaceae pathogen, B Geographical distribution of animal detected with a particular Anaplasmaceae pathogen in Africa. Cand. “E. = Candidatus “Ehrlichia
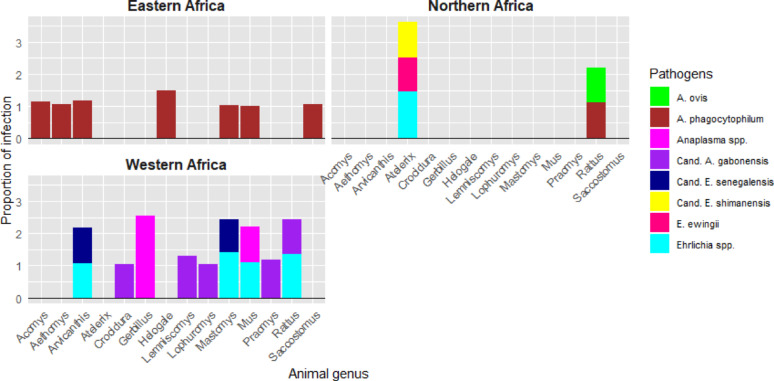
Generally, relatively few studies (14 studies) were interested to identify zoonotic Rickettsiales pathogens directly from small mammal field samples in Africa. By subregion, West Africa had the highest number of studies (5 studies), followed by East Africa with 4 studies. In West Africa, nine small mammal genera were found to be associated with various Anaplasmataceae pathogens. In East Africa, seven genera were reported; however, only a single pathogen, Anaplasma phagocytophilum, was detected across all of them. Nevertheless, none of studies from Central or Southern Africa reported the presence of Anaplasma or Ehrlichia infections in small mammals (Fig. 8).
Fig. 8.
Detail of sub-regional distribution of Anaplasmaceae pathogens detected from small mammals in Africa. Cand. A. = Candidatus “Anaplasma; Cand. E. = Candidatus “Ehrlichia
Rickettsiaceae pathogens were most commonly reported in small mammals from Southern Africa. A total of nine small mammal groups were found to carry Rickettsia species, though in varying proportions. Interestingly, Rattus spp. were detected with Rickettsia agents in all African subregions except Southern Africa, with the highest number of pathogen detections (n = 6) occurring in Northern Africa. In contrast, Rhabdomys spp. carried a relatively large number of Rickettsia species (n = 5), but were reported only in Southern Africa (Fig. 9).
Fig. 9.
Detail of sub-regional distribution of Rickettsiaceae pathogens detected from small mammals in Africa
Geographical distribution of Rickettsiales infecting ectoparasites of small mammals in Africa
Fleas were the most frequently reported ectoparasites associated with Rickettsiales infections in small mammals, particularly Rickettsia spp. The oriental rat flea (Xenopsylla cheopis) was the most commonly infected, appearing in 17 of 43 datasets from 11 studies. This was followed by the cat flea (Ctenocephalides felis) in 9 datasets from 6 studies, and Archaeopsylla spp. in 5 datasets from 3 studies. Other flea genera, including Leptopsylla spp., Echidnophaga gallinacea, and the human flea (Pulex irritans), were each reported in 3 datasets, from 3 separate studies. Ctenophthalmus spp. and Dinopsylla spp. were reported less frequently, in 2 and 1 datasets, respectively.
Fleas were mainly infected by spotted fever group rickettsiae (SFGR), reported in 24 of 43 datasets from 11 studies conducted in Tunisia, Algeria, Madagascar, Uganda, Kenya, DRC and Zambia. Among SFGR, Rickettsia felis was the most common species (17/43 datasets), followed by R. africae (4/43) and R. asembonensis (2/43).
Rickettsia typhi, the only typhus group rickettsia (TGR) reported, appeared in 9 datasets from 5 studies in Algeria, Tanzania, DRC, Egypt and Madagascar. Unspecified Rickettsia spp. were detected in 8 datasets from Uganda, Tunisia, Kenya and DRC. Additionally, two Anaplasma species (A. ovis and Anaplasma spp.) were identified in X. cheopis in a single study from Tunisia. The distribution of Rickettsiales across flea genera in Africa is illustrated in Fig. 10A and B.
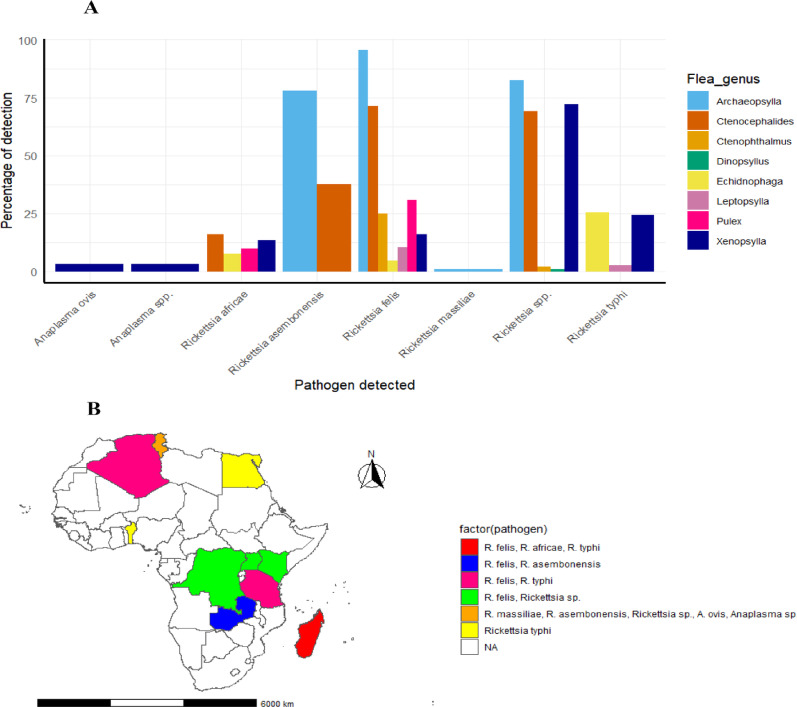
Fig. 10.
Distribution of Rickettsiales detected from various genus of fleas in Africa: A Percentage of Flea genera detected with particular Rickettsiales B Geographical distribution of fleas detected with a particular Rickettsiales pathogen in Africa
Across subregions, Eastern Africa reported the highest diversity of flea genera infected with Rickettsiales pathogens (six genera), followed by Northern Africa (five genera). In Eastern Africa, Xenopsylla spp. (particularly X. cheopis) and Ctenocephalides spp. (C. felis) were associated with the largest number of Rickettsia species. In Northern Africa, Xenopsylla spp. also dominated, alongside Archaeopsylla spp. No Rickettsiales infections were reported in fleas from Southern Africa (Fig. 11).
Fig. 11.
Detail of sub-regional distribution of Rickettsiales detected from fleas associated with small mammals in Africa
Five genera of hard ticks associated with small mammals in Africa were reported to carry Rickettsiales bacteria. Rhipicephalus was the most common genus, found in 18 of 34 datasets, followed by Haemaphysalis (7 datasets), Amblyomma (4 datasets), and Ixodes (3 datasets). Two soft tick genera were also reported: Ornithodoros, associated with Mus spp. in one study from Algeria, and Argas, reported in one study from South Africa. Ticks carrying spotted fever group rickettsiae (SFGR) were reported in 18 of 29 datasets from 11 studies across Madagascar, Benin, Tunisia, Ivory Coast, Algeria, Nigeria, South Africa and Mali.
No typhus group rickettsiae (TGR) were reported in ticks from small mammals.
Unclassified Rickettsia species (Rickettsia spp.) were detected in 11 datasets from South Africa, Nigeria, Algeria and Tunisia. Two studies also reported Anaplasma species in ticks. One from South Africa found A. bovis in Rhipicephalus ticks; another from Tunisia detected Anaplasma spp. in the same genus. Ehrlichia species were reported in two studies: E. ewingii and Ehrlichia spp. in Haemaphysalis ticks (Tunisia), and E. ruminantium in Amblyomma ticks (Mali). The distribution of Rickettsiales pathogens across tick genera in Africa is illustrated in Fig. 12A and B.
Fig. 12.
Distribution of Rickettsiales detected from various genera of Ticks in Africa: A Percentage of ticks detected with particular Rickettsiales pathogen, B Geographical distribution of ticks detected with a particular Rickettsiales pathogen in Africa
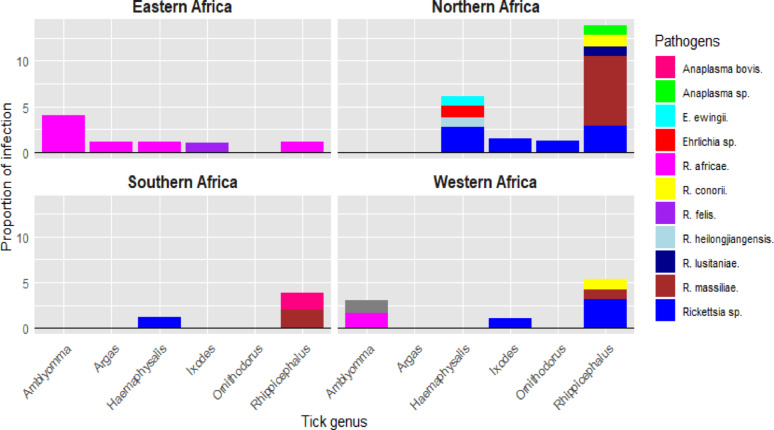
In East Africa, R. africae was common pathogen detected in 4 out of 5 genera of ticks reported. However, Amblyomma spp. was mostly with the highest proportion of infection. In North Africa, ticks were found to carry eight different Rickettsiales, with Rhipicephalus spp. hosting the majority—five pathogens in total. In Southern Africa, Rhipicephalus spp. was the only tick genus reported to be infected by both Anaplasmataceae (Anaplasma spp.) and Rickettsiaceae (R. massiliae), in ticks infesting small mammals (Fig. 13).
Fig. 13.
Detail of sub-regional distribution of Rickettsiales detected from ticks associated with small mammals in Africa
However, only two studies reported the presence of Rickettsiales bacteria from lice associated with small mammals in Africa. Rickettsia africae was reported in a single study from Madagascar to infect the lice species Haematopinus quadripertusus, Lemurpediculus verruculosus, Linognathus africanus, and L. vituli. In contrast, Rickettsia typhi was detected in Hoplopleura pacifica lice parasitizing Rattus norvegicus in Egypt. On the other side, only one study from Egypt reported the detection of Rickettsia australis from Ornithonyssus bacoti mites associated with small mammals in Africa.
Quantitative analysis
Separate meta-analysis was conducted for group Rickettsiaceae and Anaplasmaceae bacteria family identified through molecular methods in Africa for both small mammals and ectoparasite. Same analysis was also performed for Rickettsiales bacteria in 5 specific small mammals and 8 specific ectoparasites.
Rickettsiales pathogens in small mammals in Africa
Overall, the pooled prevalence of Anaplasmaceae in small mammals was higher (12.97%; 95% CI 7.79–20.82; I2 = 67.63; p < 0.0004) than that of Rickettsiaceae (Table 1). This trend was also observed when focusing only on rodents. The predictive intervals (PI), which estimate the expected range of future prevalence, were relatively narrow. For all small mammals, the PI was 2.86–42.97% for Anaplasmaceae and 0.91–17.42% for Rickettsiaceae. For rodents only, the PI ranged 3.03–36.13% and 0.9–16.34%, respectively. These narrow intervals suggest that future studies may yield similarly precise results. However, there was considerable heterogeneity in the Rickettsiaceae data (I2 >85%), indicating the need to explore potential sources of variation.
Table 1.
Meta-analysis on the molecular prevalence of Rickettsiales detected from small mammals in Africa
| Pathogen | Animal type | No. of datasets | No. of animal tested | No. of animal + Ve | Pooled prevalence (%) | 95% CI (%) | 95% PI (%) | T2(SE) | Q | I2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Rickettsiacea family | Alla | 33 | 8254 | 685 | 5.2 | 3.77–7.15 | 1.31–18.51 | 0.5 (0.38) | < 0.0001 | 87.05 |
| Rodents | 29 | 7979 | 681 | 5.13 | 3.69–7.09 | 1.35–17.62 | 0.46 (0.36) | < 0.0001 | 87.6 | |
| Shrews | 3 | 255 | 4 | 14.57 | 0.44–86.87 | 0.017–99.43 | 9.00 (10.76) | < 0.01 | 87.08 | |
| Anaplasmacea family | Alla | 12 | 424 | 59 | 12.97 | 7.79–20.82 | 2.86–42.97 | 0.6 (0.44) | 0.0004 | 67.63 |
| Rodents | 9 | 364 | 47 | 11.73 | 6.85–19.36 | 3.03–36.13 | 0.46 (0.41) | 0.0057 | 62.94 | |
| Hedgehogs | 3 | 60 | 12 | 16.48 | 3.31–53.22 | 0.81–82.67 | 1.84 (2.46) | < 0.01 | 78.57 | |
| Anaplasma spp. | Alla | 5 | 240 | 39 | 15.17 | 8-26.9 | 3.85–44.41 | 0.45 (0.48) | 0.0092 | 70.3 |
| Ehrlichia spp. | Alla | 7 | 184 | 20 | 10.35 | 4.13–23.66 | 1.11–54.38 | 1.16(1.04) | 0.0029 | 69.85 |
| Rickettsia felis | Alla | 3 | 1389 | 205 | 12 | 6.13–22.18 | 3.73–32.47 | 0.27(0.44) | 0.0214 | 73.98 |
| Rickettsia massiliae | Alla | 3 | 90 | 11 | 10.75 | 0.6-70.55 | 0.039–97.38 | 6.22 (7.21) | < 0.0001 | 89.89 |
| Rickettsia helvetica | Alla | 6 | 1401 | 213 | 14.65 | 9.09–22.75 | 6.25–30.62 | 0.16 (0.26) | 0.0783 | 59.47 |
Alla combined animal types for both rodents, shrews and hedgehogs
Within the Anaplasmaceae family, Anaplasma spp. showed a higher pooled prevalence (15.17%; 95% CI 8–26.9; I2 = 70.3; p < 0.0092) than Ehrlichia spp. (10.35%; 95% CI 4.13–23.66; I2 = 69.85; p < 0.0029). Whereas, for Rickettsiaceae, only spotted fever group rickettsiae (SFGR) were represented. R. helvetica had the highest pooled prevalence (14.65%; 95% CI 9.09–22.75; I2 = 49.47; p < 0.0783), followed by R. felis (12%; 95% CI 6.13–22.18; I2 = 73.98; p = 0.0214). Most Rickettsiales bacteria had relatively narrow PIs, supporting confidence in future estimates. However, R. massiliae showed a very wide PI (0.039–97.38%), indicating high uncertainty in future prevalence predictions based on current data (Table 1).
Rickettsiales detected in ectoparasites
At the family level, Anaplasmaceae showed a slightly higher pooled prevalence in ectoparasites (18.66%; 95% CI 5.53–47.34; I2 = 92%; p < 0.0001) than Rickettsiaceae (17.65%; 95% CI 12.41–24.48; I2 = 94.42%; p < 0.0001). Ticks carried the highest prevalence for both families. Anaplasmaceae was found in 25.43% (95% CI 4.75–70.84; I2 = 94%; p < 0.01), and Rickettsiaceae in 23.25% (95% CI 15.32–33.65; I2 = 82.33%; p < 0.0001). However, the prediction intervals (PIs) for both were wide, indicating uncertainty in future prevalence estimates. An exception was Rickettsiaceae in lice, which had a narrower PI (3.92–22.02%), suggesting more reliable estimates in future studies. Most estimates at the family level showed significant heterogeneity (I2 >80%), justifying further investigation into potential sources of variation (Table 2).
Table 2.
Meta-analysis on the molecular prevalence of Rickettsiales detected from ectoparasites of small mammals in Africa
| Pathogen | Ecto. type | No. of datasets | No. of ecto. tested | No. of ecto. +Ve | Pooled prevalence (%) | 95% CI (%) | 95% PI (%) | T2(SE) | Q | I2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Anaplasmacea family | Alla | 8 | 345 | 92 | 18.66 | 5.53–47.34 | 0.51–91.11 | 3.27 (2.60) | < 0.0001 | 92 |
| Anaplasmacea family | Ticks | 5 | 259 | 80 | 25.43 | 4.57–70.84 | 0.35–97.07 | 4.45 (4.38) | < 0.01 | 94 |
| Anaplasmacea family | Fleas | 3 | 86 | 12 | 9.81 | 0.87–57.27 | 0.095–92.58 | 4.21 (5.11) | < 0.01 | 87 |
| Rickettsiacea family | Allb | 74 | 5425 | 1194 | 17.65 | 12.41–24.48 | 0.79–85.18 | 2.77(0.74) | < 0.0001 | 94.42 |
| Rickettsiacea family | Ticks | 26 | 1082 | 214 | 23.25 | 15.32–33.65 | 3.57–71.25 | 1.08 (0.62) | < 0.0001 | 82.33 |
| Rickettsiacea family | Fleas | 43 | 4524 | 930 | 15.89 | 9.28–25.88 | 0.41–89.78 | 3.74 (1.24) | < 0.0001 | 96.14 |
| Rickettsiacea family | Lice | 5 | 460 | 44 | 9.69 | 6.04–15.19 | 3.92–22.02 | 0.18 (0.23) | 0.06 | 55.57 |
| Rickettsia spp. | Alla | 16 | 1198 | 357 | 19.14 | 9.54–34.69 | 0.96–85.29 | 2.49 (1.43) | < 0.0001 | 95.02 |
| Rickettsia spp. | Ticks | 8 | 490 | 115 | 27.31 | 14.51–45.34 | 4.9-73.24 | 0.86 (0.8) | < 0.01 | 85.81 |
| Rickettsia spp. | Fleas | 8 | 708 | 242 | 12.98 | 3.51–37.99 | 0.18–92.53 | 4.56(3.49) | < 0.01 | 96.73 |
| Rickettsia massiliae | Alla | 8 | 296 | 25 | 21.57 | 6.15–53.59 | 0.66–91.97 | 3.08 (2.75) | < 0.0001 | 82.85 |
| Rickettsia massiliae | Ticks | 7 | 205 | 24 | 30.55 | 8.96–66.29 | 1.14–94.37 | 2.86 (2.79) | < 0.01 | 82.18 |
| Rickettsia felis | Alla | 18 | 1252 | 483 | 20.45 | 8.34–42.08 | 0.34–95.1 | 4.58 (2.26) | < 0.0001 | 95.73 |
| Rickettsia felis | Fleas | 17 | 1241 | 472 | 21.68 | 8.67–44.68 | 0.36–95.46 | 4.58 (2.34) | < 0.0001 | 95.78 |
| Rickettsia africae | Allb | 14 | 1041 | 142 | 14.17 | 9.69–20.26 | 4.5-36.66 | 0.36 (0.25) | < 0.0001 | 69.84 |
| Rickettsia africae | Ticks | 6 | 238 | 63 | 41.53 | 13.53–76.33 | 2.22–95.68 | 2.49 (2.56) | < 0.01 | 79.29 |
| Rickettsia africae | Fleas | 4 | 389 | 36 | 10.29 | 6.88–15.12 | 5.84–17.50 | 0.05 (0.18) | 0.28 | 21.9 |
| Rickettsia africae | Lice | 4 | 414 | 43 | 10.63 | 6.92–15.99 | 4.98–21.25 | 0.12 (0.19) | 0.1 | 51.64 |
| Rickettsia typhi | Fleas | 10 | 1281 | 85 | 8.23 | 3.47–18.28 | 0.58–57.88 | 1.72 (1.21) | < 0.0001 | 90.6 |
Alla combined ectoparasites types for both ticks and fleas, Allb combined ectoparasite types for both ticks, fleas and lice
In ticks, R. africae had the highest pooled prevalence (41.53%; 95% CI 13.53–76.33; I2 = 79.29%; p < 0.01), followed by R. massiliae (30.55%; 95% CI 8.96–66.29; I2 = 82.85%; p < 0.0001) and Rickettsia spp. (27.31%; 95% CI 14.51–45.34; I2 = 94.66%; p < 0.0001).
However, these results had very wide PIs—from 4.9 to 73.24% to 0.35–97.07%—indicating a high level of uncertainty for future estimates based on current data. Whereas, in fleas, Rickettsia spp. showed the highest pooled prevalence (27.6%; 95% CI 8.56–60.84; I2 = 96.61%; p < 0.01), followed by R. felis (20.45%; 95% CI 8.34–42.08; I2 = 95.23%; p < 0.0001) and R. typhi with the lowest (8.23%; 95% CI 3.47–18.28; I2 = 90.6%; p < 0.0001). Among these, R. typhi had the narrowest PI (5.84–17.5%), suggesting more certainty in future prevalence estimates for this species. Across all ectoparasite-pathogen combinations, substantial heterogeneity (I2 >75%) was present. This highlights the need to explore potential underlying factors that may influence variation in pooled prevalence estimates.
Moderators analysis
Subgroup analysis indicated that, sample size (6/11 pathogen groups) followed by molecular detection techniques (4/11 pathogen groups) and sampling country (4/11 pathogen groups) were the most potential underlying determinants associated with estimated prevalence of pathogens detected from ectoparasites. Whereas, estimated prevalence of pathogens detected from small mammals especially R. massiliae and R. helvetica were mostly associated with detection techniques, sampling country and region, and animal genus (Table 3).
Table 3.
Demonstration of statistical significance (p-values) of the moderators selected for subgroup analysis
| Pathogen | Animal/ecto.a type | No. of datasets | Sample source | Detection techn.b | Sampling region | Sampling country | Animal/ecto. genus | Sample size | Pathogens | Year of publication |
|---|---|---|---|---|---|---|---|---|---|---|
| For small mammals | ||||||||||
| Anaplasmaceae | All animals | 12 | 0.3611 | 0.4629 | 0.7911 | 0.7911 | 0.8763 | 0.9356 | 0.6731 | 0.2306 |
| Rodents | 9 | N/A | 0.9543 | 0.643 | 0.643 | 0.232 | 0.4432 | 0.6768 | 0.0181 | |
| Rickettsiaceae | All animals | 33 | 0.6275 | 0.8254 | 0.8716 | 0.9657 | 0.41 | 0.4222 | < 0.0001 | 0.2921 |
| Rodents | 29 | N/A | 0.7867 | 0.8023 | 0.9215 | 0.5863 | 0.2103 | 0.0003 | 0.2332 | |
| Anaplasma | All animals | 5 | N/A | 0.1829 | 0.1829 | 0.1829 | 0.3857 | 0.9554 | N/A | 0.0012 |
| Ehrlichia | All animals | 7 | 0.1767 | 0.1695 | 0.1767 | 0.1767 | 0.4649 | 0.294 | N/A | 0.1767 |
| For ectoparasites | ||||||||||
| Rickettsiaceae | Alla | 75 | 0.079 | 0.0287 | 0.6828 | 0.1772 | 0.1722 | 0.0017 | 0.0043 | |
| Ticks | 26 | N/A | 0.9585 | 0.8727 | 0.4912 | 0.2187 | 0.0471 | 0.5751 | 0.0617 | |
| Fleas | 43 | N/A | 0.0269 | 0.6642 | 0.3691 | 0.2064 | 0.0243 | 0.0169 | 0.3358 | |
| Anaplasmaceae | Alla | 8 | 0.4729 | 0.3102 | < 0.0001 | < 0.0001 | 0.9081 | 0.9762 | 0.0316 | |
| Anaplasma spp. | Alla | 4 | 0.1726 | 0.7631 | < 0.0001 | < 0.0001 | 0.1726 | 0.0296 | N/A | |
| Ehrlichia spp. | Alla | 3 | N/A | 0.5307 | 0.5307 | 0.5307 | 0.5307 | 0.5307 | N/A | |
| Rickettsia spp. | Alla | 18 | 0.4201 | 0.0223 | 0.6826 | 0.0268 | 0.5263 | 0.001 | 0.0012 | 0.0002 |
| R. massiliae | Alla | 8 | 0.0676 | 0.2902 | 0.9157 | 0.3534 | 0.0676 | 0.0553 | N/A | 0.9573 |
| R. felis | Alla | 18 | 0.5455 | 0.1918 | 0.1882 | 0.0751 | 0.1363 | 0.3629 | 0.0436 | |
| R. africae | Alla | 14 | 0.0382 | 0.0424 | 0.0424 | 0.0424 | 0.3618 | 0.0373 | ||
| R. typhi | Alla | 10 | 0.4471 | 0.5179 | 0.4981 | 0.4005 | 0.6588 | 0.1202 | 0.5225 | |
a ectoparasites, b molecular detection method, All animal = All small mammals combined, Alla All ectoparasites combined
Pooled estimated molecular prevalence of the specific targeted Anaplasmacea or Rickettsiacea pathogen detected from different genera of ectoparasites and small mammals are summarized in Table 4. We combined the significant variables into several meta-regression models in the subgroup analysis. The best-fitting models, those explaining the greatest proportion of heterogeneity, are presented in Table 5, under the “formula” column. The test of moderators was significant for most pathogen groups, indicating that the selected variables do indeed influence the prevalence of these groups. However, residual heterogeneity remained high (> 75%) for many pathogens, except for Anaplasma species in small mammals. This suggests that the estimated prevalence is also affected by factors not accounted for in our study.
Table 4.
Estimated pooled prevalence of specific Rickettsiales pathogen detected from specific ectoparasites or small mammal genera
| Animal/ Ecto.a genus |
Anaplasmacea | Anaplasma sp. | Ehrlichia sp. | Rickettsiacea | Rickettsia sp. | R. massiliae | R. africae | R. felis | R. typhi | R. conorii | Rasembonensis. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| For small mammals | |||||||||||
| Rhabdomys | 7.69 (5.55–10.54) | ||||||||||
| Rattus | 9.98 (5.45–17.58) | 4.35 (2.15–8.61) | |||||||||
| Mastomys | 4.71 (0.34–41.61) | ||||||||||
| Lemniscomys | 7.61 (0.025–99.48) | ||||||||||
| Crocidura | 7.13 (0.069–89.57) | ||||||||||
| Atelerix | 16.48 (3.31–53.22) | ||||||||||
| For ectoparasites | |||||||||||
| All ticks | 25.43 (4.57–70.84) | 46.09 (0.79–98.93) | 14.25 (2.73–49.59) | 23.25 (15.32–33.65) | 27.31 (14.54–45.34) | 30.55 (8.96–66.29) | 41.52 (13.53–76.33) |
15.93 (6.09–35.65) |
|||
| Rhipicephalus | 46.09 (0.79–98.93) | 20.20 (9.99–36.62) | 19.24 (0.54–91.32) | 30.55 (8.96–66.29) | |||||||
| Haemaphysalis | 21.41 (10.40-39.01) | 10.39 (1.15–53.60) | |||||||||
| Ixodes | 16.52 (1.72–69.11) | ||||||||||
| All flea | 18.52 (10.94–29.61) | 27.60 (8.56–60.84) |
21.68 (8.67- 44.68) |
9.19 (3.75–20.82) | 59.57 (20.68–89.28) | ||||||
| Xenopsylla | 8.77 (4.85–15.35) | 24.94 (5.47–65.63) |
6.15 (2- 17.36) |
5.65 (2-14.95) |
|||||||
| Ctenocephalides | 40.99 (22.02–63.07) |
39.03 (13.13- 73.06) |
|||||||||
| Echidnophaga | 25.76 (2.13–84.69) | ||||||||||
| Pulex | 9.13 (1.17–46.04) |
8.08 (0.29–72.53) |
|||||||||
| Archaeopsylla | 70.58 (43.63–88.15) |
88.17 (49.58–98.26) |
|||||||||
| All lice | 9.69 (6.04–15.19) | ||||||||||
Table 5.
Meta-regression test results
| Pathogen | Formula | (I2), % | (R2), % | (p-value) |
|---|---|---|---|---|
| Small mammals | ||||
| Anaplasma (all) | Year of publication | 0 | 100 | 0.0015 |
| Rickettsiacea (all) | Detected pathogen | 79.18 | 58.39 | 0.0002 |
| Rickettsiacea (Rodents) | Detected pathogen | 84.41 | 47.37 | 0.0087 |
| Ectoparasites | ||||
| Rickettsiacea (ticks) | Sample size | 88.1 | 12.97 | 0.0471 |
| Rickettsiacea (fleas) | Sample size + Pathogens detected | 95.35 | 18.03 | 0.0334 |
| Rickettsia spp. (all) | Detection techn. + Sampling country + Sample size + Detected pathogen + Year of publication | 95.01 | 37.21 | 0.0297 |
| Rickettsia felis | Year of publication | 79.39 | 61.89 | 0.0003 |
Discussion
The emerging and re-emerging of zoonotic diseases pose substantial challenges to public and veterinary health systems in Africa, where limited resources and infrastructure magnify the burden of infectious diseases. The increased trend insinuates the need to investigate the possible vectors and reservoir hosts for potential zoonotic transmission routes to humans. The presence of particular zoonotic Anaplasma, Ehrlichia or Rickettsia group of bacterial agents in small mammals or their respective ectoparasites increases the risks of pathogen transmission. This systematic review confirms that small mammals and their ectoparasites in Africa harbor a diverse range of potentially zoonotic Rickettsiales bacteria, particularly from the Rickettsiaceae and Anaplasmaceae families. The findings underscore the importance of understanding ecological and epidemiological factors influencing host-vector-pathogen dynamics especially in areas with frequent human–animal interactions [71, 72].
This work evaluates systematically the reported data on important potentially zoonotic Rickettsiaceae and Anaplasmaceae pathogens detected from small mammals and their respective ectoparasites in Africa from 2000 to 2024. We focused on the presence of pathogens, infestation status, prevalence of infection estimates and epidemiological risks of transmission to humans. Our analysis of 37 studies from 17 African countries reveals notable geographic variation in the reporting of Rickettsiales infections. Eastern Africa had the highest number of studies (13/37 studies), likely reflecting a long-standing research interest driven by historical occurrences of plague, murine typhus, and other rodent-borne zoonoses in Tanzania, Kenya, Uganda and Madagascar [73, 74]. The prominence of R. typhi in this region, despite its relatively low pooled prevalence in our meta-analysis, warrants concern due to its overlap with endemic plague caused by Yersinia pestis [55, 57, 60, 66]. This co-circulation has important implications for clinical diagnosis and public health response, as both pathogens present with non-specific febrile symptoms and share flea vectors [23]. In addition, the history of Hantavirus in Western Africa [75] and Boutonneuse fever (Mediterranean spotted fever) caused by R. conorii in Northern Africa [76], might be the influencing factor more research interest in rodents for these regions as observed in this study.
Our analysis showed that, SFGR are the most reported Rickettsiales pathogen represented by R. felis, R. massiliae and R. africae. Rickettsia felis and R. africae emerged as the most frequently detected spotted fever group rickettsiae (SFGR), particularly in Eastern Africa. The high prevalence of R. felis in fleas and R. africae in ticks suggests that flea- and tick-borne rickettsioses may be underdiagnosed contributors to febrile illnesses in this region [46]. Therefore, R. felis and R. africae should be considered as very important zoonotic SFGR in Eastern Africa countries potentially vectored by fleas and ticks infesting small mammals, respectively. On the other hand, R. massiliae was frequently reported from Northern Africa especially from Rhipicephalus spp. ticks. This is the zoonotic emerging rickettsial pathogen [77], which has been reported to cause rifampin-resistant cases [78]. So, it is worth to note that, occurrence of this zoonotic pathogen with high pooled prevalence ~ 30% in Rhipicephalus ticks should seriously taken into consideration especially in Northern and Western African countries. Rhipicephalus spp. ticks and Xenopsylla spp. fleas were frequently identified as competent vectors across multiple small mammal hosts. Additionally, Rhipicephalus spp. was the only tick genus carrying both Rickettsiaceae and Anaplasmaceae pathogens in some subregions, supporting its designation as a key bridge vector at the wildlife–livestock–human interface.
This study revealed that, pooled estimate of prevalence for most Rickettsiaceae pathogens in specific small mammals genus group was relatively low ranged from ~ 4–8%. Kemani et al. [52]. justified that, Rickettsia pathogens induce temporary infection in animals, in which the pathogen remain viable for only respective short time then it is eliminated while the animal become immune for another pathogen of the same kind. Thus, small mammals especially frequent reported ones (Rattus, Mastomys, Rhabdomys, Lemniscomys, Crocidura and Atelerix) should stand to be highly potential route of zoonotic transmission to human regardless of their low pooled estimate of prevalence. This is due to their high interactions along animal-livestock-human interfaces in Africa.
This study highlighted that, ticks, fleas, mites and lice were the main blood-sucking ectoparasites of small mammals identified with Rickettsiales bacteria. Mites had much higher pooled mean intensity per individual small mammal followed by ticks and least in fleas. The trend of results was in congruent with their morphological and physiological characteristics of these ectoparasites. Mites are comparatively less active in movement and commonly found in high abundance in the furs and skin of their host. Ticks always found in larval (nymph) stage and attach on the skin to suck blood so to molt into respective next stage of their life cycle. While fleas have developed hind limbs for jumping whenever they sense disturbance so they are always found off-host [49]. However, various field evidenced studies which focused on the ectoparasitic infestation of small mammals put forth the gradient trends of abundance as mites > fleas > lice > ticks [15, 79].
Analysis revealed that, fleas—particularly Xenopsylla spp. and Ctenocephalides spp.—were more consistently linked to pathogen transmission especially in Eastern Africa. The high prevalence of Rickettsiaceae in fleas especially for East Africa may be justified by high contact between fleas, wildlife, domestic animals and human with high circulating Rickettsia species (R. typhi, R. felis and R. africae) [46, 55, 57, 58, 66]. The ecological behavior of fleas (frequent host-switching, persistent off-host survival) enhances their capacity as vectors. Xenopsylla spp. (especially X. cheopis) plays a considerable role in transmission of several zoonotic bacteria including Rickettsiales pathogens including R. africae; the agent of African tick bite fever (ATBF), R. felis; associated as agent of human fever of unknown origin in Western Africa and Europe [19, 80] and TGR (R. typhi; the agent of endemic murine typhus in Africa [23]). The vector competence of X. cheopis to carry multiple Rickettsiales pathogens, supports its central role in zoonotic transmission cycles.
As revealed in analysis of this study, Xenopsylla spp. also reported to infest large number of small mammals’ groups. This concrete the fact that, Xenopsylla spp. especially X. cheopis is the most competent vector for rickettsial bacteria and host generalist with significant implication in vector-borne disease pathogen transmission has been suggested in various studies [49, 56, 67]. The study conducted by Torina et al. [81]. , highlighted the potence of Xenopsylla spp. to be co-infected by different member of bacterial groups. The cat flea (Ctenocephalides felis) on the other hand was reported to be the main vector of flea-borne spotted fever rickettsiosis (by R. felis) in Africa and elsewhere [48, 49, 59]. Hence, these two species of fleas should be considered as important vectors for zoonotic Rickettsiaceae in Africa. However, the pathogenicity and main risk factors as main vectors of rickettsial agents for these flea species was not well studied in Africa. Although their transovarial and transstadial transmission rickettsial pathogens to their offspring is suggested among the factor [82–84].
Our analysis also demonstrated that the tick genera Rhipicephalus and Haemaphysalis harbored a higher diversity of Rickettsiales than other ectoparasites. The higher prevalence of Anaplasma spp. in Rhipicephalus ticks (46.09%) compared to Haemaphysalis spp. (21.41%) underscores their differing roles in pathogen ecology. The results are in congruent with reviewed results by Cossu et al. [85]. which suggest Rhipicephalus spp. as the competent vector for Rickettsiaceae especially R. massiliae and R. conorii in Africa. On the other hand, Amblyomma spp. was suggested as important vector transmitting various important zoonotic Rickettsiaceae and Anaplasmaceae in higher animals such as livestock [86] although, it revealed to be less common reported in small mammals. Under this review only one group of small mammals the hedgehogs (Atelerix spp., Setifer setosus and Echinops telfairi) was reported to be infested by Amblyomma spp [43, 46]. This is most probably due to the fact that, hedgehogs are relatively larger enough for large size tick like Amblyomma spp. to attach and get required amount of blood for survival compared to most small mammals found in Africa. Therefore, these two species of ticks (Rhipicephalus spp. and Haemaphysalis spp.) should be considered as a serious potential vector to transmit zoonotic Rickettsiales from small mammals along animal-human interfaces in Africa. However, the ecological preferences, life cycles, and vectorial capacity of these ticks remain poorly studied in the African context, representing a major research gap. Future work should integrate fine-scale ecological data including land use, climate variability, and host abundance to model potential hotspots of Rickettsiales transmission.
Generally, host specificity also plays a key role in shaping pathogen transmission risk. Some ectoparasites, such as Xenopsylla cheopis and Rhipicephalus sanguineus, exhibit low host specificity and parasitize a wide range of small mammals. This ecological generalism increases the likelihood of cross-species pathogen transmission and spillover to humans. In contrast, species like Ctenocephalides felis tend to infest specific hosts (e.g., domestic cats) but still serve as competent vectors for zoonotic pathogens such as R. felis. The tendency of Rickettsiaceae to cause transient infections in reservoir hosts, as noted by Kemani et al. [52], suggests that low prevalence estimates may still reflect significant transmission potential particularly in species with high contact rates at the human–animal interface.
From a One Health perspective, this review points to several underexplored environmental drivers that may shape the spatial and temporal patterns of Rickettsiales transmission. Deforestation and agricultural encroachment alter habitat structure, often increasing rodent abundance and bringing humans into closer contact with infected wildlife. Urbanization and poor housing infrastructure, particularly in rapidly expanding peri-urban areas, create ideal conditions for flea proliferation and rodent infestation. Climate change, by shifting temperature and precipitation patterns, likely affects the distribution and seasonal activity of both ectoparasites and their hosts—potentially expanding the transmission window for Rickettsiales pathogens. These ecological pressures may explain the observed regional heterogeneity in pathogen prevalence and should be incorporated into future surveillance models.
Diagnostic capacity also influences our understanding of disease dynamics. Historically, serological methods like the Weil-Felix test dominated Rickettsiales diagnostics but are limited by low specificity due to cross-reactivity [87, 88]. Thus, they are considered highly sensitive but less specific. Some advanced serology tests are current available in the market for laboratory diagnostics of Rickettsiaceae or Anaplasmaceae such as Indirect immunofluorescence assay (IFA) and microimmunofluorescence (MIF), however its cost implication and need for expertise is still limited in most endemic areas. Additionally, the cross reactivity for close related rickettsial groups are still common challenge even [88]. In contrast, molecular techniques, especially qPCR and sequencing, offer greater precision and have now become standard in most recent studies. However, the reliance on short gene fragments in qPCR can still lead to misclassification. Our analysis revealed that all the 37 included studies used molecular methods for the identification of different groups of Rickettsiales employing different modalities. Only two studies under this review employed qPCR as the only screening and confirmatory test [60]. To counteract this shortcoming, specific conversional PCR must be performed after qPCR to attest its specificity before taking the sample to downstream processes such as gene sequencing as justified by most authors of studies in this review. Techniques of PCR incorporated by sequencing made it easy to identify, confirm, classify and characterize the pathogen to species, subspecies or strain levels.
Our review highlights the value of combining qPCR with multi-locus sequence typing (MLST) and sequencing to enhance taxonomic resolution and detect emerging or novel Rickettsiales genotypes. As justified by two authors of studies from Kenya [48] and South Africa [51] under this review revealed qPCR incorporated by multi locus sequence typing (MLST) is good technique to elucidate the certainty of Rickettsiales group identification. This is because, most Anaplasmaceae and Rickettsiaceae groups are genomically closely related, hence usage of MLST ensures the exploration of all possible member groups of pathogens in the respective sample. For example, MLST enables to discrimination between R. felis-like (Candidatus “R. assembonensis”) from genetically close similar R. felis in Kenya [48].
One of the key limitations of this review is the presence of wide confidence and predictive intervals (PIs) in some of the pooled prevalence estimates, largely resulting from small sample sizes in several of the included studies. Additionally, sample size emerged as a significant contributor to heterogeneity in effect sizes during the subgroup (moderator) analysis, consistent with findings from other meta-analyses where study size influences between-study variability [89, 90]. These wide intervals indicate reduced statistical precision and underscore the uncertainty surrounding the true prevalence of Rickettsiales infections in small mammals and their ectoparasites. Consequently, the generalizability of certain findings may be limited, particularly for pathogens or host-vector associations reported in only one or two studies [91]. This underscores the need for larger, more geographically diverse studies to improve the reliability and applicability of future prevalence estimates.
Although studies were included from multiple African regions, there was uneven geographical representation, with underreporting or absence of data from several countries in Central and Western Africa. This limits the generalizability of the findings across the continent. Consequently, the reported prevalence may vary significantly if similar studies were conducted in other regions of the continent. Furthermore, we did not account for several potential determinants that could influence prevalence estimates or act as confounding factors. These include biological variables such as growth stage and sex of both host animals and their ectoparasites, as well as environmental factors such as vegetation, soil properties, and climatic conditions (e.g., temperature, humidity, and rainfall).
A major observation toward reviewed studies in this work is the lack of standardization in determining the prevalence of Rickettsiales pathogens in African small mammals and their ectoparasites. Notably, the molecular methods employed in the included studies varied considerably, particularly in the selection of gene targets. This inconsistency hindered a more detailed comparative analysis of the genetic markers used for pathogen detection. Additionally, most included studies reported pathogen detection via qPCR and sequencing but did not evaluate vector competence (i.e., ability of ectoparasites to transmit the pathogen), which limits conclusions about actual transmission risk.
On the other side, randomization and justification of sample sizes were rarely considered in the reviewed studies. As shown in supplementary Table S4 of the AXIS tool, fewer than half of the studies (16/37, or 43.2%) satisfied question 6, which assesses whether randomization was applied, while none of the studies fulfilled the criteria for question 3, which evaluates whether the sample size was justified. Therefore, we strongly recommend that future studies incorporate randomization in their sampling strategies whenever possible, as this approach enhances the reliability of findings and reduces the risk of selection bias.
Conclusion and recommendation
In this review, we systematically demonstrated the broad ecological distribution and public health significance of Rickettsiales bacteria in African small mammals and their ectoparasites. The findings highlight Rickettsia felis, R. africae, R. conorii, R. massiliae, and R. helvetica as significant SFG Rickettsia, while R. typhi belongs to the typhus group; both groups should be considered serious zoonotic pathogens. Similarly, Anaplasma phagocytophilum and Ehrlichia ruminantium emerge as critical zoonotic Anaplasmataceae pathogens carried and maintained by small mammals, warranting targeted intervention in Africa. Additionally, small mammal species such as Rattus spp., Mastomys spp., Mus spp., Rhabdomys spp., and hedgehogs (Atelerix spp.) should be regarded as serious potential reservoirs of Rickettsiales pathogens in Africa. Among ectoparasites, fleas (Xenopsylla spp., Ctenocephalides spp.) and ticks (Rhipicephalus spp., Haemaphysalis spp., Amblyomma spp.) are likely key competent vectors associated with small mammals for Rickettsiales pathogen transmission. Finally, quantitative real-time PCR combined with multi-locus sequence typing (MLST) has proven to be the most effective approach for the surveillance and identification of Rickettsiales pathogens in Africa.
We emphasize the importance of adopting a One Health framework for coordinated monitoring of zoonotic Rickettsiales in wildlife, livestock, vectors, and human populations especially in areas undergoing rapid socio-ecological transformation. Strengthening molecular diagnostic capacity especially the combination of PCR technologies with MLST, expanding geographic coverage, and improving data sharing between public health, veterinary, and environmental sectors are key priorities for surveillance and outbreak preparedness.
Ultimately, Rickettsiales infections in Africa remain neglected but potentially significant public health threats. A shift toward proactive, cross-sectoral strategies is essential for timely detection, risk mitigation, and targeted interventions. Future efforts should prioritize high-risk regions, emerging pathogens, and the ecological interfaces where spillover is most likely to occur.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are deeply grateful to everyone involved in the development and improvement of this review. We also extend our sincere thanks to the sponsors, African Centre of Excellence for Innovative Rodent Pest Management and Biosensor Technology Development (ACE II IRPM and BTD), for their support in making this study possible. Special appreciation goes to the staff of the Department of Wildlife Management at Sokoine University of Agriculture for their approval and for hosting the study. We would like to express our special thanks to staff of SUA Agriculture National Library (SNAL) represented by Mr. Jabil Jabil for their esteemed technical support especially during systematic article searching.
Abbreviations
- ATBF
African tickborne fever
- AXIS
Appraisal Tool for Cross-Sectional Studies
- cPCR
Conventional polymerase chain reaction
- DRC
Democratic Republic of the Congo
- ELISA
Enzyme linked immunosorbent assay
- IFA
Indirect immunofluorescence assay
- MIF
Microimmunofluorescence
- MLST
Multi locus sequence typing
- PCR
Polymerase chain reaction
- PI
Prediction interval
- PICO
Population Intervention Comparison Outcome guide model
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- qPCR
Qualitative real-time polymerase chain reaction
- SFGR
Spotted fever group Rickettsia
- TGR
Typhus group Rickettsia
- WFT
Weil-Felix test
Author contributions
Conceptualization of research idea, methodology preparation and original draft preparation: V.T.M.; critical appraisal of included studies: V.T.M, C.M.L., M.I.O., A.S.K., R.H.M, A.W.M., G.M., L.L.M.; data curation and analysis: V.T.M., M.I.O., G.M.; review and editing: C.M.L., M.I.O., A.S.K., R.H.M, A.W.M., G.M., L.L.M.; supervision: G.M., L.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by the Africa Centre of Excellence for Innovative Rodent Pest Management and Biosensor Technology Development (ACE II IRPM and BTD) at the Institute of Pest Management of the Sokoine University of Agriculture, Tanzania (ACEII-credit no. 5799-TZ).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Risk assessment for conducting this study was submitted to and approved by the Research Ethical Committee and Decision Board of Sokoine University of Agriculture, SUA/PFC/D/2020/0001/14, issued on 22 December 2020.
Consent for publication
“Not applicable”.
Competing interests
The authors declare no competing interests.
Footnotes
References [34–70] are cited in the Table S1 of supplementary materials. These are articles selected and included for this particular review manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Zoonotic disease: emerging public health threats in the Region. 2009.
- 2.Raza A, Ahmad S, Ahmad A, Zain-UI-Abedin M, Channo A, Subhan A, et al. Zoonotic diseases: emerging threats to public health and livestock production. IJAB. 2023;Zoonosis 1:74–88. [Google Scholar]
- 3.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nat Publishing Group. 2008;451:990–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Royal Soc. 2001;356:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asante J, Noreddin A, El Zowalaty M. Systematic review of important bacterial zoonoses in Africa in the last decade in light of the ‘one health’ concept. Pathogens. 2019;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird BH, Mazet JAK. Detection of emerging zoonotic pathogens: an integrated one health approach. Annu Rev Anim Biosci. 2018;6:121–39. [DOI] [PubMed] [Google Scholar]
- 7.Namusisi S, Mahero M, Travis D, Pelican K, Robertson C, Mugisha L. A descriptive study of zoonotic disease risk at the human-wildlife interface in a biodiversity hot spot in South Western Uganda. PLoS Negl Trop Dis. 2021;15:e0008633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odhiambo CO, Oguge NO. Patterns of rodent pest distribution in a maize cropping system in the Kenyan Rift Valley. Rats, mice and people : rodent biology and management. 2003;:217–9.
- 9.Mgode GF, Katakweba AS, Mhamphi GG, Fwalo F, Bahari M, Mdangi M, et al. Prevalence of leptospirosis and toxoplasmosis: a study of rodents and shrews in cultivated and fallow land, Morogoro rural district, Tanzania. Tanzan J Health Res. 2014;16:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Belmain SR. Rats and Human Health in Africa: Proceedings of an international workshop on rodent-borne diseases and the RatZooMan research project. In: Ratzooman workshop. 2006. pp. 1–46.
- 11.Makundi RH, Massawe AW, Mulungu LS. Ecological considerations for management of rodent pests in Tanzania. Manage Sel Crop Pests Tanzan 2006; March 2014:224–39.
- 12.Wiethoelter AK, Beltrán-Alcrudo D, Kock R, Mor SM. Global trends in infectious diseases at the wildlife-livestock interface. Proc Natl Acad Sci USA. 2015;112:9662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viteri MC, Hadly EA. Spatiotemporal impacts of the anthropocene on small mammal communities, and the role of small biological preserves in maintaining biodiversity. Front Ecol Evol. 2022;10:916239. [Google Scholar]
- 14.Wheelis M. Biological warfare at the 1346 siege of Caffa. Emerg Infect Dis. 2002;8:971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shilereyo M, Magige F, Ranke PS, Ogutu JO, Røskaft E. Ectoparasite load of small mammals in the Serengeti ecosystem: effects of land use, season, host species, age, sex and breeding status. Parasitol Res. 2022;121:823–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.khosravani M. The fauna and perspective of rodentia ectoparasites in Iran relying on their roles within public health and veterinary characteristics. J Parasitic Dis. 2018;42:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihalca AD, Dumitrache MO, Sándor AD, Magda C, Oltean M, Györke A, et al. Tick parasites of rodents in romania: host preferences, community structure and geographical distribution. Parasites Vectors. 2012;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahdar M, Vazirianzadeh B, Rointan ES, Amraei K. Identification of collected ectoparasites of rodents in the West of Khuzestan Province (Ahvaz and Hovizeh), southwest of Iran. Asian Pac J Trop Disease. 2015;5:627–31. [Google Scholar]
- 19.Guccione C, Colomba C, Iaria C, Cascio A. Rickettsiales in the WHO European region: an update from a one health perspective. Parasites Vectors. 2023;16:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGinn J, Lamason RL. The enigmatic biology of rickettsiae: recent advances, open questions and outlook. Pathogens Disease. 2021;79:ftab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosso F, Tagliapietra V, Baráková I, Derdáková M, Konečný A, Hauffe HC, et al. Prevalence and genetic variability of Anaplasma phagocytophilum in wild rodents from the Italian alps. Parasites Vectors. 2017;10:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svitálková Z, Haruštiaková D, Mahríková L, Berthová L, Slovák M, Kocianová E, et al. Anaplasma phagocytophilum prevalence in ticks and rodents in an urban and natural habitat in South-Western Slovakia. Parasites Vectors. 2015;8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dill T, Dobler G, Saathoff E, Clowes P, Kroidl I, Ntinginya E, et al. High Seroprevalence for typhus group rickettsiae, Southwestern Tanzania. Emerg Infect Dis. 2013;19:317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peniche-Lara G, Dzul-Rosado K, Pérez-Osorio C, Zavala-Castro J. Rickettsia Typhi in rodents and R. felis in fleas in Yucatán as a possible causal agent of undefined febrile cases. Rev Inst Med Trop Sao Paulo. 2015;57:129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee W, Seong H, Kim JH, Choi H, Kim JH, Ahn JY, et al. A case of African Tick-Bite fever in a returning traveler from Southern Africa. Infect Chemother. 2022;54:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raoult D, Fournier PE, Fenollar F, Jensenius M, Prioe T, De Pina JJ, et al. Rickettsia africae, a Tick-Borne pathogen in travelers to Sub-Saharan Africa. N Engl J Med. 2001;344:1504–10. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. 2009. [PMC free article] [PubMed]
- 28.Nishikawa-Pacher A. Research questions with PICO: A universal mnemonic. Publications. 2022;10:21. [Google Scholar]
- 29.African Union. Member States | African Union. Member states. 2024. https://au.int/en/member_states/countryprofiles2. Accessed 21 Oct 2024.
- 30.Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6:e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis with R: A Hands-On Guide. 1st edition. Boca Raton: Chapman and Hall/CRC; 2021.
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 34.Harrison A, Bown KJ, Horak IG. Detection of Anaplasma Bovis in an undescribed tick species collected from the Eastern rock Sengi Elephantulus Myurus. J Parasitol. 2011;97:1012–6. [DOI] [PubMed] [Google Scholar]
- 35.Selmi R, Belkahia H, Dhibi M, Abdelaali H, Lahmar S, Ben Said M, et al. Zoonotic vector-borne bacteria in wild rodents and associated ectoparasites from Tunisia. Infect Genet Evol. 2021;95:105039. [DOI] [PubMed] [Google Scholar]
- 36.Ouarti B, Sall M, Ndiaye EHI, Diatta G, Zan Diarra A, Berenger JM, et al. Pathogen detection in Ornithodoros Sonrai ticks and invasive house mice Mus musculus domesticus in Senegal. Microorganisms. 2022;10:2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolo AO, Collins NE, Brayton KA, Chaisi M, Blumberg L, Frean J, et al. Anaplasma phagocytophilum and other Anaplasma spp. In various hosts In the Mnisi community, Mpumalanga province, South Africa. Microorganisms. 2020;8:1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosha ET, Kuria JKN, Otiende M, Lekolool I. Molecular detection of Anaplasma phagocytophilum in small mammals and infesting ticks in Laikipia county, Kenya. Veterinary Med Int. 2024;2024:5575162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangombi JBP, Granjon L, Flirden F, Kane M, Niang Y, Davoust B, et al. Molecular survey of Rodent-Borne infectious agents in the Ferlo region, Senegal. Genes (Basel). 2023;14:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young HS, McCauley DJ, Dirzo R, Nunn CL, Campana MG, Agwanda B, et al. Interacting effects of land use and climate on rodent-borne pathogens in central Kenya. Phil Trans R Soc B. 2017;372:20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balti G, Galon C, Derghal M, Souguir H, Guerbouj S, Rhim A, et al. Atelerix algirus, the North African hedgehog: suitable wild host for infected ticks and fleas and reservoir of Vector-Borne pathogens in Tunisia. Pathogens. 2021;10:953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangombi JB, N’dilimabaka N, Lekana-Douki J-B, Banga O, Maghendji-Nzondo S, Bourgarel M, et al. First investigation of pathogenic bacteria, protozoa and viruses in rodents and shrews in context of forest-savannah-urban areas interface in the City of Franceville (Gabon). PLoS ONE. 2021;16:e0248244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diarra AZ, Kone AK, Doumbo Niare S, Laroche M, Diatta G, Atteynine SA, et al. Molecular detection of microorganisms associated with small mammals and their ectoparasites in Mali. Am J Trop Med Hyg. 2020;103:2542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diagne C, Galan M, Tamisier L, d’Ambrosio J, Dalecky A, Bâ K, et al. Ecological and sanitary impacts of bacterial communities associated to biological invasions in African commensal rodent communities. Sci Rep. 2017;7:14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahmana H, Granjon L, Diagne C, Davoust B, Fenollar F, Mediannikov O. Rodents as hosts of pathogens and related zoonotic disease risk. Pathogens. 2020;9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehlers J, Krüger A, Rakotondranary SJ, Ratovonamana RY, Poppert S, Ganzhorn JU, et al. Molecular detection of rickettsia spp., borrelia spp., Bartonella spp. And yersinia pestis in ectoparasites of endemic And domestic animals in Southwest Madagascar. Acta Trop. 2020;205:105339. [DOI] [PubMed] [Google Scholar]
- 47.Nziza J, Tumushime JC, Cranfield M, Ntwari AE, Modrý D, Mudakikwa A, et al. Fleas from domestic dogs and rodents in Rwanda carry Rickettsia asembonensis and Bartonella tribocorum. Med Vet Entomol. 2019;33:177–84. [DOI] [PubMed] [Google Scholar]
- 48.Jiang J, Maina AN, Knobel DL, Cleaveland S, Laudisoit A, Wamburu K et al. Molecular detection of Rickettsia felis and Candidatus Rickettsia Asemboensis in Fleas from Human Habitats, Asembo, Kenya. Vector-Borne and Zoonotic Diseases. 2013;13:550–8. [DOI] [PMC free article] [PubMed]
- 49.Bai Y, Osikowicz LM, Kosoy MY, Eisen RJ, Atiku LA, Mpanga JT, et al. Comparison of zoonotic bacterial agents in fleas collected from small mammals or Host-Seeking fleas from a Ugandan region where plague is endemic. Am Soc Microbiol. 2017;2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moonga LC, Hayashida K, Nakao R, Lisulo M, Kaneko C, Nakamura I, et al. Molecular detection of Rickettsia felis in dogs, rodents and Cat fleas in Zambia. Parasit Vectors. 2019;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Essbauer S, Hofmann M, Kleinemeier C, Wölfel S, Matthee S. Rickettsia diversity in Southern africa: A small mammal perspective. Ticks Tick-borne Dis. 2018;9:288–301. [DOI] [PubMed] [Google Scholar]
- 52.Kamani J, Baneth G, Gutiérrez R, Nachum-Biala Y, Mumcuoglu KY, Harrus S. Coxiella burnetii and Rickettsia conorii: two zoonotic pathogens in peridomestic rodents and their ectoparasites in Nigeria. Ticks Tick-borne Dis. 2018;9:86–92. [DOI] [PubMed] [Google Scholar]
- 53.Bitam I, Parola P, Matsumoto K, Rolain JM, Baziz B, Boubidi SC, et al. First molecular detection of R. conorii, R. aeschlimannii, and R. massiliae in ticks from Algeria. Ann N Y Acad Sci. 2006;1078:368–72. [DOI] [PubMed] [Google Scholar]
- 54.Reeves WK, Loftis AD, Szumlas DE, Abbassy MM, Helmy IM, Hanafi HA, et al. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp). Exp Appl Acarol. 2007;41:101–7. [DOI] [PubMed] [Google Scholar]
- 55.Eneku W, Erima B, Byaruhanga AM, Cleary NG, Atim G, Tugume T et al. Molecular detection and characterization of emerging pathogens of Rickettsia felis and felis-like organisms from peri-domestic fleas in uganda. Preprint. In Review; 2023.
- 56.Loftis AD, Dasch GA, Szumlas DE, Helmy IM, Moriarity JR, Reeves WK, et al. Surveillance of Egyptian fleas for agents of public health significance: anaplasma, bartonella, coxiella, ehrlichia, rickettsia, and yersinia pestis. Am J Trop Med Hyg. 2006;75:41–8. [PubMed] [Google Scholar]
- 57.Rakotonanahary RJL, Harrison A, Maina AN, Jiang J, Richards AL, Rajerison M, et al. Molecular and serological evidence of flea-associated typhus group and spotted fever group rickettsial infections in Madagascar. Parasit Vectors. 2017;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasoamalala F, Parany MNJ, Rahajandraibe S, Rakotomanga MN, Ramihangihajason T, Soarimalala V, et al. High rickettsial diversity in rodents and their ectoparasites from the central highlands of Madagascar. J Med Entomol. 2022;59:667–74. [DOI] [PubMed] [Google Scholar]
- 59.Bitam I, Baziz B, Kernif T, Harrat Z, Parola P, Raoult D. Molecular detection of Rickettsia Typhi and Rickettsia felis in fleas from Algeria. Clin Microbiol Infect. 2009;15:255–6. [DOI] [PubMed] [Google Scholar]
- 60.Leulmi H, Socolovschi C, Laudisoit A, Houemenou G, Davoust B, Bitam I, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leulmi H, Aouadi A, Bitam I, Bessas A, Benakhla A, Raoult D, et al. Detection of Bartonella tamiae, Coxiella burnetii and rickettsiae in arthropods and tissues from wild and domestic animals in Northeastern Algeria. Parasites Vectors. 2016;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khaldi M, Socolovschi C, Benyettou M, Barech G, Biche M, Kernif T, et al. Rickettsiae in arthropods collected from the North African Hedgehog (Atelerix algirus) and the desert Hedgehog (Paraechinus aethiopicus) in Algeria. Comp Immunol Microbiol Infect Dis. 2012;35:117–22. [DOI] [PubMed] [Google Scholar]
- 63.Halajian A, Palomar AM, Portillo A, Heyne H, Luus-Powell WJ, Oteo JA, Investigation. of Rickettsia, Coxiella burnetii and Bartonella in ticks from animals in South Africa. Ticks and Tick-borne Diseases. 2016;7:361–6. [DOI] [PubMed]
- 64.Berrelha J, Briolant S, Muller F, Rolain J-M, Marie J-L, Pagés F, et al. Rickettsia felis and Rickettsia massiliae in Ivory coast, Africa. Clin Microbiol Infect. 2009;15:251–2. [DOI] [PubMed] [Google Scholar]
- 65.Reeves WK, Szumlas DE, Moriarity JR, Loftis AD, Abbassy MM, Helmy IM, et al. Louse-borne bacterial pathogens in lice (Phthiraptera) of rodents and cattle from Egypt. J Parasitol. 2006;92:313–8. [DOI] [PubMed] [Google Scholar]
- 66.Thomas CA, Abdul AS, Massawe AW, Makundi RH, Machang RS, Kessy ST. Prevalence of Rickettsia Typhi in rodent fleas from areas with and without previous history of plague in Mbulu district. Tanzania. 2020;14:65–70. [Google Scholar]
- 67.Laudisoit A, Falay D, Amundala N, Akaibe D, de Bellocq JG, Van Houtte N, et al. High prevalence of Rickettsia Typhi and Bartonella species in rats and fleas, kisangani, Democratic Republic of the congo. Am J Trop Med Hyg. 2014;90:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lafri I, Leulmi H, Baziz-Neffah F, Lalout R, Mohamed C, Mohamed K, et al. Detection of a novel Rickettsia sp. in soft ticks (Acari: Argasidae) in Algeria. Microbes Infect. 2015;17:859–61. [DOI] [PubMed] [Google Scholar]
- 69.Yessinou RE, Adehan S, Hedegbetan GC, Cassini R, Mantip SE, Farougou S. Molecular characterization of Rickettsia spp., Bartonella spp., and Anaplasma phagocytophilum in hard ticks collected from wild animals in Benin, West Africa. Trop Anim Health Prod. 2022;54:306. [DOI] [PubMed]
- 70.Masakhwe C, Linsuwanon P, Kimita G, Mutai B, Leepitakrat S, Yalwala S, et al. Identification and characterization of Orientia Chuto in trombiculid chigger mites collected from wild rodents in Kenya. J Clin Microbiol. 2018;56:e01124–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Childs JE, Mackenzie JS, Richt JA, editors. Wildlife and emerging zoonotic diseases: the biology, circumstances and consequences of Cross-Species transmission. Berlin Heidelberg: Springer Science + Business Media;: Springer-; 2007. [Google Scholar]
- 72.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife - Threats to biodiversity and human health. Science’ Compass. 2000;287:443–9. [DOI] [PubMed] [Google Scholar]
- 73.Davis DH. Plague in Africa from 1935 to 1949; a survey of wild rodents in African territories. Bull World Health Organ. 1953;9:665–700. [PMC free article] [PubMed] [Google Scholar]
- 74.Eisen RJ, Akol A, Atiku LA, Amatre G, Gage KL, Enscore RE, et al. Flea diversity and infestation prevalence on rodents in a Plague-Endemic region of Uganda. Am J Trop Med Hyg. 2009;81:718–24. [DOI] [PubMed] [Google Scholar]
- 75.Klempa B, Witkowski PT, Popugaeva E, Auste B, Koivogui L, Fichet-Calvet E, et al. Sangassou virus, the first hantavirus isolate from africa, displays genetic and functional properties distinct from those of other Murinae-associated hantaviruses. J Virol. 2012;86:3819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rovery C, Brouqui P, Raoult D. Questions on mediterranean spotted fever a century after its discovery. Emerg Infect Dis J. 2008;14. [DOI] [PMC free article] [PubMed]
- 77.Chao L-L, Robinson M, Liang Y-F, Shih C-M. First detection and molecular identification of Rickettsia massiliae, a human pathogen, in Rhipicephalus sanguineus ticks collected from Southern Taiwan. PLoS Negl Trop Dis. 2022;16:e0010917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beati L, Roux V, Ortuño A, Castella J, Porta FS, Raoult D. Phenotypic and genotypic characterization of spotted fever group rickettsiae isolated from Catalan Rhipicephalus sanguineus ticks. J Clin Microbiol. 1996;34:2688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Babyesiza WS, Mpagi J, Ssuuna J, Akoth S, Katakweba A. Ectoparasite fauna of rodents and shrews with their spatial, temporal, and dispersal along a degradation gradient in Mabira central forest reserve. J Parasitol Res. 2023;2023:7074041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Angelakis E, Mediannikov O, Parola P, Raoult D. Rickettsia felis: the complex journey of an emergent human pathogen. Trends Parasitol. 2016;32:554–64. [DOI] [PubMed] [Google Scholar]
- 81.Torina A, Blanda V, Antoci F, Scimeca S, D’Agostino R, Scariano E et al. A Molecular Survey of Anaplasma spp., Rickettsia spp., Ehrlichia canis and Babesia microti in Foxes and Fleas from Sicily. Transbound Emerg Dis. 2013;60:125–30. [DOI] [PubMed]
- 82.Azad AF, Sacci JB, Nelson WM, Dasch GA, Schmidtmann ET, Carl M. Genetic characterization and transovarial transmission of a typhus-like Rickettsia found in Cat fleas. Proc Natl Acad Sci USA. 1992;89:43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fongsaran C, Jirakanwisal K, Tongluan N, Latour A, Healy S, Christofferson RC, et al. The role of cofeeding arthropods in the transmission of Rickettsia felis. PLoS Negl Trop Dis. 2022;16:e0010576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gutiérrez R, Krasnov B, Morick D, Gottlieb Y, Khokhlova IS, Harrus S. Bartonella infection in rodents and their flea ectoparasites: an overview. Vector-Borne Zoonotic Dis. 2015;15:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cossu CA, Collins NE, Oosthuizen MC, Menandro ML, Bhoora RV, Vorster I, et al. Distribution and prevalence of anaplasmataceae, rickettsiaceae and Coxiellaceae in African ticks: A systematic review and Meta-Analysis. Microorganisms. 2023;11:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madison-Antenucci S, Kramer LD, Gebhardt LL, Kauffman E. Emerging Tick-Borne diseases. Clin Microbiol Rev. 2020;33:e00083–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stewart AG, Stewart AGA. An update on the laboratory diagnosis of rickettsia spp. Infect Pathogens. 2021;10:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Wiley; 2009.
- 91.Sedgwick P. Meta-analyses: what is heterogeneity? BMJ. 2015;350:h1435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].