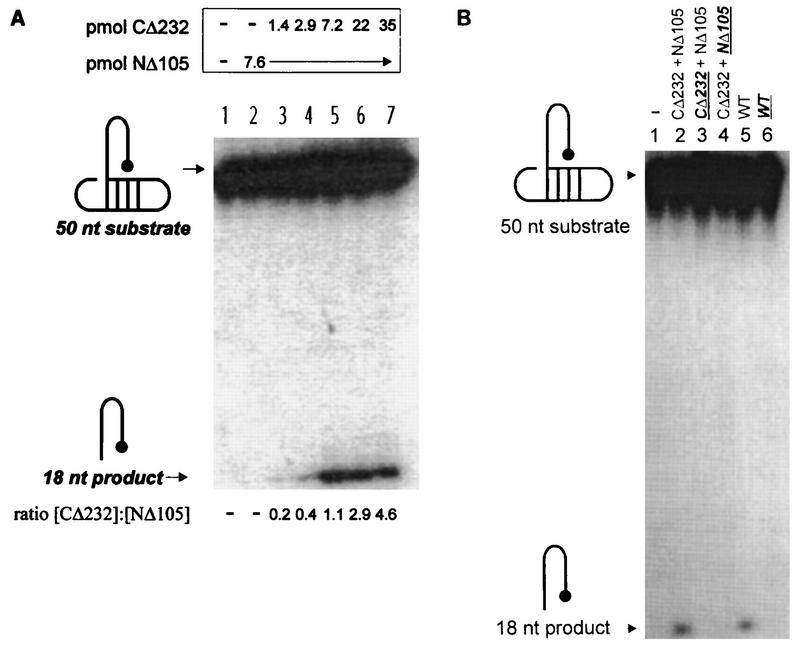
FIG. 4.
Influence of the HHCC domain, residue C209, and the LTR 5′-ss tail on NΔ105. The structures of the 5′-32P-labeled untailed dumbbell substrate (7440, 5′-TGAAAGCGTAAGCTTTCAACCTGCGTAAGCAGGTAGACCGCAAGGTCT-3′) and the reaction product of unimolecular disintegration are indicated on the left of each panel. (A) Requirement of the HHCC domain in trans to NΔ105 for unimolecular disintegration of an untailed dumbbell disintegration substrate. Lane 1 contains a control buffer reaction. The molar quantities of the proteins and their ratios in each reaction are respectively indicated above and below each lane. (B) Effect of NEM alkylation of residue C209 or the HHCC domain on productive interactions with an untailed dumbbell substrate. Individual M-MuLV IN proteins and complementation protein pairs are indicated as in Fig. 2 and 3. NEM-treated samples are indicated in bold and are underlined.