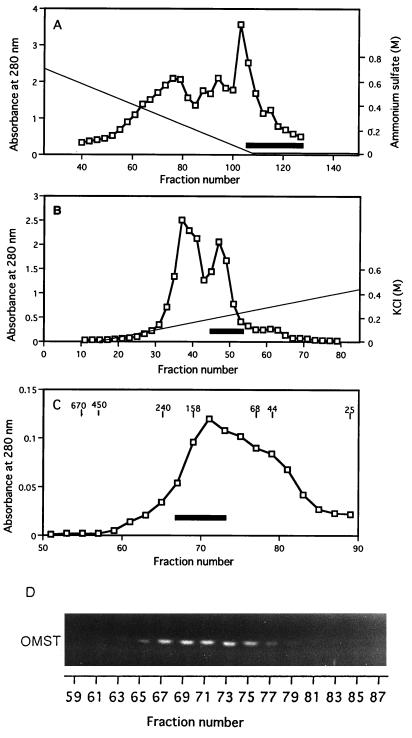
FIG. 2.
MT-I chromatographies. (A) First phenyl-Sepharose fractionation. Cytosol fraction added with ammonium sulfate was loaded onto a phenyl-Sepharose column, and the column effluent after a linear gradient of (NH4)2SO4 (solid line) followed by washing with the solution without (NH4)2SO4 was assayed for protein (absorbance at 280 nm; open squares) and MT-1 activity (open squares) by measuring the production of OMST from DMST. The volume of each fraction was 2 ml, and the solid bar indicates active fractions collected. (B) DEAE-Sepharose ion-exchange chromatography. Proteins were eluted by successive linear gradient washes with KCl. The volume of each fraction was 1 ml, and the solid bar indicates active fractions collected. (C) Sephacryl S-300 gel filtration chromatography. After rechromatography of the pooled fractions with phenyl-Sepharose, active fractions were collected, concentrated, and applied to a Sephacryl S-300 column. The solid line indicates active fractions collected. Molecular standards are indicated in kilodaltons, and the volume of each fraction was 1 ml. (D) Fluorescence photographs showing the production of OMST from DMST by each fraction after Sephacryl S-300 gel filtration. The NEM-treated cytosol fraction was added into each reaction mixture containing an aliquot of each fraction.