Like the Earth’s water, nitrogen compounds cycle through the air, aquatic systems, and soil. But unlike water, these compounds are being injected into the environment in ever increasing quantities. In doing so, we are altering the global nitrogen cycle, causing possible grave impacts on biodiversity, global warming, water quality, human health, and even the rate of population growth in developing nations.
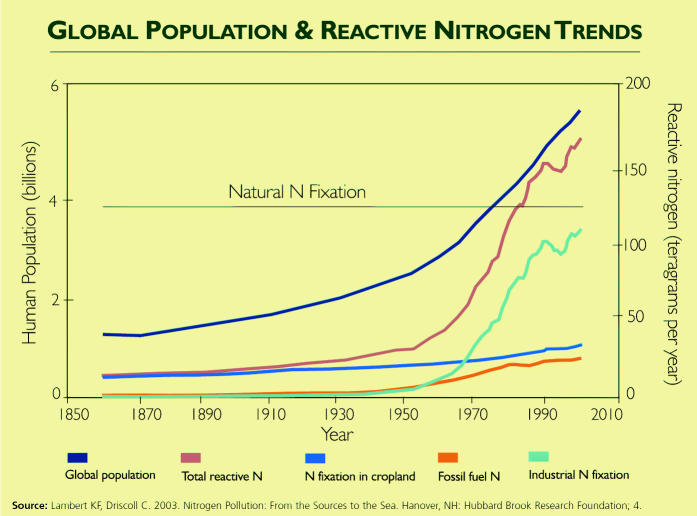
In a world surrounded by nitrogen, you would think there’s always been plenty to go around and that perhaps a little more wouldn’t matter. But having enough of the right kind of nitrogen—reactive nitrogen that has been “fixed,” or converted from the nonreactive N2 form—determines such fundamentals of life as the extent of plant growth, which in turn determines to a large extent the dynamics of the world’s food supply. During the twentieth century, mankind has produced increasingly more reactive nitrogen, intentionally as fertilizer and unintentionally as a by-product of combusting fossil fuels.
Although carbon dioxide may get more press, “the nitrogen cycle has been altered more than any other basic element cycle,” says John Aber, vice president for research and public service at the University of New Hampshire. And now, he says, humans are adding more reactive nitrogen to the global nitrogen cycle than all other sources combined. Yet, reactive nitrogen is hardly all bad. The use of nitrogen fertilizer is critical to feeding the world’s hungry, say researchers including University of Virginia environmental sciences professor James Galloway. The question, then, is how do we manage nitrogen responsibly?
A Natural History of Nitrogen
Everything that lives needs nitrogen. But most atoms of nitrogen—which represents 78% of the atmosphere—are bound tightly in pairs as N2. Most organisms can’t break the powerful triple bond of the N2 molecule’s two atoms. For plants to grow and animals to thrive, they need the element in a reactive fixed form that is bonded to carbon, hydrogen, or oxygen, most often as organic nitrogen compounds (such as amino acids), ammonium (NH4), or nitrate (NO3). Animals get their reactive nitrogen from eating plants and other animals somewhere along the food chain. And plants get reactive nitrogen from the soil or water.
Lightning accounts for some naturally occurring reactive nitrogen—worldwide each year, lightning fixes an estimated 3–10 teragrams (Tg), the usual measurement unit for discussing the global nitrogen cycle. The energy that lightning generates converts oxygen and nitrogen to nitric oxide (NO), which oxidizes to nitrogen dioxide (NO2), then to nitric acid (HNO3). Within days the HNO3 is carried to the ground in rain, snow, hail, or other atmospheric deposition. This source of reactive nitrogen is important to areas in which nitrogen-fixing plants are scarce.
Most naturally occurring reactive nitrogen comes from nitrogen fixation by bacteria, including cyanobacteria and specialized bacteria such as those in the genus Rhizobium, which most often live symbiotically in plants such as peas, beans, and alfalfa. According to a literature review published in the April 2003 issue of BioScience by Galloway and colleagues, experts believe natural, nonagricultural organisms fix 100–300 Tg of nitrogen per year on the land surfaces of the Earth, although most estimates tend toward the lower end.
Farmers eventually learned to increase the levels of reactive nitrogen in their soil using plants that have nitrogen-fixing bacterial symbionts, but their resources were limited: at the beginning of the twentieth century they could rotate with nitrogen-fixing crops such as legumes, or add naturally occurring fertilizers such as manure, guano, and nitrate mineral deposits mined in Chile. At this point, according to the BioScience review, humans were producing about 15 Tg of reactive nitrogen per year.
Around this time, however, German scientists Fritz Haber and Carl Bosch developed a way to convert nonreactive atmospheric nitrogen to ammonia, the reactive compound that forms the base of nitrogen fertilizer. Currently, the Haber-Bosch process is used to produce about 100 Tg of reactive nitrogen per year worldwide, most of which is used to produce nitrogen fertilizer. Food grown with this fertilizer feeds some 2 billion people, estimates Vaclav Smil, a professor of geography at the University of Manitoba, writing in the July 1997 issue of Scientific American.
The past 15 years have seen a huge explosion in the amount of reactive nitrogen that humans have produced and injected into the environment, according to a report on relationships between the global nitrogen cycle and human health in volume 1, issue 5 (2003) of Frontiers in Ecology and the Environment by Alan Townsend, an assistant professor of ecology and evolutionary biology at the University of Colorado, and colleagues. Human production of reactive nitrogen is currently estimated to be about 170 Tg per year, write Galloway and colleagues in the BioScience review, and the global use of nitrogen fertilizers is increasing by about 15 Tg per year. The ratio of anthropogenic to natural reactive nitrogen creation is likely to increase with population increases, Galloway says. More mouths to feed will require both more reactive nitrogen fertilizers in the ground and the clearing of unspoiled, nitrogen-fixing lands to make farmland.
Human Sources of Reactive Nitrogen
Where does all this human-generated reactive nitrogen come from? The largest contributor is nitrogen fertilizer. As of 2000, about 100 Tg of reactive nitrogen were released each year from nitrogen fertilizer spread on farmlands around the world, according to the BioScience review. As modern farming methods have been increasingly adopted, so has the rate at which nitrogen is being fixed, with much of the increase coming in developing countries, according to Townsend and colleagues in Frontiers in Ecology and Environment. In their BioScience review, Galloway and colleagues write that widespread cultivation of nitrogen-fixing crops such as legumes has added another approximately 40 Tg of reactive nitrogen.
Burning of biomass—the use of wood for fuel and the clearing of forests and grasslands for agriculture—converts another 40 Tg or so. Draining wetlands allows organic material in the soil to oxidize, and clearing land of vegetation for crops can free reactive nitrogen from soils. These sources contribute about 10 and 20 Tg, respectively, according to an article in the Spring 1997 Issues in Ecology by a team led by Peter Vitousek, a professor of population and resource studies at Stanford University.
Fossil fuel combustion also contributes to the reactive nitrogen load. “It’s not just agriculture that’s changing the nitrogen cycle,” says Michael Mallin, a research professor at the University of North Carolina at Wilmington’s Center for Marine Science. “Urbanization is doing it in a big way. Cities are full of cars. Cars release nitrogen oxides [NOx; the collective term for NO and NO2]. It goes up into the air and comes down as somebody else’s problem.” By fixing atmospheric nitrogen and releasing reactive nitrogen that otherwise would be sequestered indefinitely in fuels, fossil fuel combustion contributes about 20 Tg of reactive nitrogen globally each year.
Very few parts of the Earth now lack their own regional sources of reactive nitrogen pollution, says David Tilman, a professor of ecology at the University of Minnesota. “Agricultural expansion has really taken over the whole world,” he says. “The rates of fertilization per hectare—the nitrogen added per hectare—are not that different. Not just among the seven or eight most industrialized nations, but even among nations that are not industrial giants, the agricultural side has really pursued nitrogen fertilization.” Galloway adds that nitrogen pollution is distributed globally not just by wind and water but also by ship and truck: “International commerce is a major way of shipping reactive nitrogen around the world,” he says.
As a result, Galloway says, there are significant sources of polluting reactive nitrogen in just about any corner of the Earth, with the unfortunate exception of much of Africa, which although spared much direct nitrogen pollution, is also deprived of the sorely needed fertilizer. Currently Asia, Europe, and North America account for almost 90% of human-generated reactive nitrogen, Galloway says. European countries such as the Netherlands (where long-term nitrogen fertilizer use and many concentrated animal farms have created perhaps the world’s most nitrogen-saturated area) and Germany have long shown the effects of nitrogen pollution. In the Netherlands, for example, extreme reactive nitrogen levels have changed the Dutch countryside’s characteristic heathlands to grasslands. But over the next 50 years, Galloway says, the developing world’s growing dependence on nitrogen fertilizers, rising population densities, and adoption of gasoline-powered vehicles are all likely to result in increases in nitrogen-related environmental and human health impacts.
A Vicious Cycle?
“The nitrogen cycle has changed on a global scale to a remarkable extent, but the rate at which that plays out locally is hugely variable,” says Townsend. “There are major hot spots at all of the industrialized nations of the world. We’re seeing incredible increases [in reactive nitrogen use/production and resulting pollution] in the United States, much of Europe, and much of Asia and China now. There are areas there, for example, that are seeing deposition from the atmosphere that is ten times or more what it was prior to human activity.”
Some of this reactive nitrogen is, of course, put to good use, Townsend says. Nitrogen fertilizers can take credit for reductions in starvation and malnutrition in many parts of the world, especially in Asia in the last decade. In fact, Smil writes in the March 2002 issue of the Swedish journal Ambio that “for at least a third of humanity in the world’s most populous countries the use of [nitrogen] fertilizers makes the difference between malnutrition and adequate diet.”
But as nitrogen levels continue to rise, Townsend says, the net health effects become increasingly negative. Furthermore, says Galloway, reactive nitrogen can not only impact many different ecosystems, but a single atom also can make mischief repeatedly, unlike most better recognized pollutants. “If you put a molecule of NOx in the atmosphere from fossil fuel combustion or a molecule of ammonium on an agricultural field as a fertilizer,” he explains, “you have a whole series, or cascade, of effects that goes from acid rain to particle formation in the atmosphere, decreasing visibility and causing impacts on human health, acid rain, soil and stream acidification, coastal eutrophication, decreasing biodiversity, human health issues in groundwater, and nitrous oxide [N2O] emissions to the atmosphere, which impact the greenhouse effect and stratospheric ozone.”
Nitrogen in the Air
The effects of reactive nitrogen on ozone are profound, wreaking havoc at every elevation. “In areas like the northeastern United States, because we have more automobiles than agriculture, our major contribution to global nitrogen cycling is oxidized forms of nitrogen,” Aber says. NOx, which can form from the application of nitrogen fertilizers, burning of biomass, and combustion of fossil fuels, is an important contributor to the formation of smog and ground-level ozone. “That’s [the Northeast’s] most important form of air pollution,” Aber says.
High concentrations of NOx, which are common in urban areas with their high car populations, can produce low-lying ozone, which in turn can cause or worsen asthma, cough, reactive airways disease, respiratory tract inflammation, and chronic respiratory disease. High levels of NOx can also worsen viral infections such as the common cold. In addition to ground-level sources, where denitrification (the conversion of reactive nitrogen to N2) in soil also produces some N2O, aircraft inject NOx directly into the atmosphere.
At mid-altitudes, N2O acts as a green-house gas, with each molecule absorbing about 200 times as much outgoing radiation as carbon dioxide. And although at low altitudes reactive nitrogen increases ozone, at very high altitudes it actually destroys ozone. In the stratosphere, ultraviolet light breaks N2O apart, producing NO, which in turn acts as a catalyst to break down ozone. Destroying ozone in the stratosphere, of course, allows more ultraviolet light to reach the Earth’s surface, resulting in more skin cancers—an article in the 30 March 1998 International Journal of Climatology by Rajaram P. Kane, a senior scientist at the Brazilian National Institute for Space Research, says that reductions in ozone suggest a 10–20% increase in ultraviolet-B radiation, which would “explain a 20–40% rise in skin cancer in the human population since the 1970s.”
The effects of N2O can persist for decades, with a residence time of 120 years in the atmosphere, says Robert Howarth, a professor of ecology and environmental biology at Cornell University. “It plays a big role in catalyzing the destruction of ozone in the stratosphere,” he explains. “It’s a greenhouse gas, and it’s a pretty potent greenhouse gas—it’s the longest-lived greenhouse gas in the atmosphere.” Once in the atmosphere, other nitrogen gases such as NOx and ammonia can also generate particulates that are small enough to penetrate deep into the lungs, contributing to cardiovascular diseases, respiratory diseases, asthma, reduced lung function, and overall mortality.
In spite of the severity of these effects, Howarth says, there is little understanding among the public of nitrogen’s role in public health, global warming, or much else. “Everyone on the street is well aware of ground-level ozone and that it is a serious health issue,” he says. “The average person on the street does not know that ozone pollution is caused by nitrogen pollution. If you did not have the nitrogen pollution, you would not have the ozone pollution.” Other indirect health effects of nitrogen pollution include promotion of the conditions favorable to cholera and the breeding conditions for the types of mosquitoes that carry West Nile virus, malaria, and encephalitis.
Other experts point to a lack of recognition—in U.S. policy-making circles, at least—of the role of reactive nitrogen in producing acid rain. Not all NOx stays aloft, says Aber. “In contact with moisture in the atmosphere, it turns into nitric acid, which is the nitrogen component of acid rain,” he says. In industrialized areas of the United States, nitric acid has become an increasingly significant component of acid raid, says Gene Likens, director of the Institute of Ecosystem Studies in Mill-brook, New York. “Our long-term studies at Hubbard Brook Experimental Forest—the longest continuous measurement of precipitation and stream-water chemistry in the world—clearly indicate that there is a major change under way,” he says. In 1963, when the studies began, he says, sulfuric acid contributed about 70% of total acidity of rain, and nitric acid was about 15%. Currently sulfuric acid is about 50%, and nitric acid is about 40%. “We project that if the current trends continue, nitric acid will become the dominant acid in eastern North America by about 2012,” Likens says. “And yet we’ve focused all of our regulations primarily on reducing sulfur.”
Nitrogen in the Water
If any aspect of nitrogen pollution has a high public and policy profile, it’s the effects of excess nutrients on bodies of water, especially in coastal areas. “Because of its high solubility, nitrate quickly escapes to down below the root zone of an agricultural field or forest and into groundwater,” says Donald Boesch, a professor of marine science and president of the University of Maryland’s Center for Environmental Science. “That makes it difficult and expensive to control.”
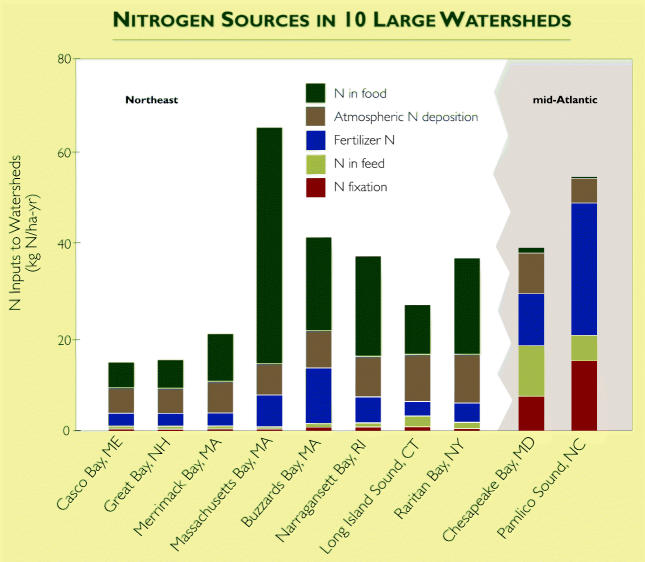
Reactive nitrogen—whether from animal-raising facilities, manufactured fertilizer, septic systems, or other sources—has raised nitrate concentrations in the waterways of most industrialized nations. In Norway, nitrate concentrations in 1,000 lakes doubled in less than a decade. Rivers in the northeastern United States and in much of Europe have increased 10- to 15-fold in the last 100 years.
Where nitrate loading to bays and costal zones increases (rivers tend to be less affected), it can provide such a steady source of nutrients that algae bloom uncontrollably. When the algae die, they sink and decompose, which draws oxygen from the water. If too much oxygen is removed, the water body develops a “dead zone”—an area that can no longer support finfish, shellfish, or most other aquatic life. Perhaps the best-known dead zone is that found in the Gulf of Mexico, which is fed by the nitrate-rich Mississippi River and fluctuates in size from 3,000 to 8,000 square miles. There are also oxygen-starved areas in the Baltic Sea, the Adriatic Sea, the Gulf of Thailand, the Yellow Sea, and the Chesapeake Bay.
Boesch notes that scientists were saying as far back as 1987 that 40% of the nitrogen coming into the system needed to be removed. But so far, he says, programs to reduce reactive nitrogen in the Chesapeake Bay haven’t significantly improved the bay’s health. And although rivers are generally less susceptible to such algal blooms and oxygen losses, Mallin has found similar effects in North Carolina’s blackwater streams, so called because they are rich in organic matter. “Regardless of what we add [nitrate, ammonia, or urea from livestock], it will stimulate algae growth in these black-water streams,” he says.
Reactive nitrogen can also infiltrate drinking water, as nitrates from nitrogen fertilizers and runoff from livestock find their way into streams, rivers, lakes, and groundwater. In the United States, Townsend says, as much as 20% of ground-water sources may exceed the U.S. and World Health Organization limits of 10 parts per million for nitrates. This concentration is also exceeded in many other parts of the world. High concentrations of nitrates can cause methemoglobinemia—or “blue baby disease”—in infants. In blue baby disease, nitrate ions weaken the blood’s capacity to carry oxygen. Epidemiological studies have also linked nitrates to reproductive problems and some cancers, including increased risks for bladder and ovarian cancers at concentrations below 10 parts per million.
Nitrogen in the Soil
As with water and air, reactive nitrogen builds up in soil. There’s a limit, however, to how much nitrogen plants can use. When soil reaches a point at which plants can’t use additional nitrogen, it’s said to be “saturated.” And saturated soil, in theory at least, will shed any additional nitrogen introduced to it. But that nitrogen doesn’t leave unaccompanied. “When it leaches out of the system,” says Townsend, “it takes other nutrients with it, so it ends up acidifying the soil, and it takes things like magnesium and calcium out into the water. And you end up with a very unbalanced system.”
If it’s true that saturated soil immediately passes additional nitrogen, rather than denitrifying it, that could be bad news for the near future, says Howarth, with all that excess nitrogen flowing straight to ground-water, rivers, streams, and seas. However, he says, we have a very poor understanding of what is actually happening. “If the nitrogen is accumulating in soil, it could be a temporary phenomenon until it saturates the ability to store it. Then we have a much bigger problem,” he says. “If it is being denitrified, on the other hand, that’s more of a steady-state process, and it can probably continue to do that.”
Townsend says some scientists had hoped that excess reactive nitrogen levels might actually reduce greenhouse gases by stimulating plant growth, which locks up carbon dioxide. But, he says, “It doesn’t seem likely that it’s going to play a dominant role.” Although the jury is still out, Tilman adds, “there isn’t very good evidence that nitrogen deposition actually does lead to increased carbon removal and storage.”
Although more reactive nitrogen means more growth, it also changes which of the species in an ecosystem thrive. For example, in grasslands that received increased nitrogen, Tilman says, “the species composition changed to plants that had litter that decomposed more quickly. And because it decomposed more quickly, there was actually no net storage of carbon with added nitrogen.”
On the surface it could seem as though additional nutrition might at least help struggling ecosystems thrive. In fact, however, reactive nitrogen can disrupt an ecosystem’s delicate balance. “From the 1850s on, we’ve known that the addition of nutrients to terrestrial ecosystems causes changes in which species are there and causes a loss of diversity in those systems,” says Tilman. “Under the highest rates of agriculturally driven nitrogen that we’ve seen, there’s a very strong effect [on biodiversity loss].” Recent field studies in Great Britain—reported by Open University Earth scientist Carly J. Stevens and colleagues in the 19 March 2004 issue of Science—have confirmed that biodiversity decreased as unaided nitrogen deposition increased in a sample of 68 grasslands. Tilman’s experimental work in which nitrogen was added to ecosystems shows similar results, he says.
Regaining Control
Reducing the amount of reactive nitrogen that is added to the environment is critical, Galloway says. Of the nitrogen that is created to sustain food production, only about 2–10% enters the human mouth, depending on the region. The rest, he says, is lost to the environment: “Unless an equivalent amount is denitrified back to molecular N2, then that means reactive nitrogen is accumulating in the environment, in the atmosphere, in the groundwater, in the soils, in the biota.”
Some solutions are at best long-term, or simply unlikely. If many of the world’s meat-eaters were to switch to a largely vegetarian diet, Townsend says, farmers could plant far less nitrogen-stoked grain, most of which goes to animal feed and sweeteners. But meat consumption in the United States and Asia is rising rather than falling. It has also been suggested that symbiotic bacteria could someday be genetically engineered to bestow grains directly with nitrogen-fixing capability.
A more practical, low-tech, low-cost solution is to improve the ways farmers rotate crops and fertilize their lands, says Stanford University Earth science professor Pamela Matson. In the American Midwest, for example, it’s common for farmers to fertilize their fields in the fall. Winter snow and spring thaw wash away far more fertilizer than stays in the soil. Many farmers in all regions that have especially unpredictable weather intentionally overfertilize, she says, rather than run the risk of running short of nutrients in a year in which conditions would otherwise result in a bumper crop. The alternative, which Matson says some farmers manage well, is to add exactly the right amount of fertilizer exactly when it is needed.
In an effort to better understand the problems associated with changes in the nitrogen cycle and reduce their negative impacts, the Swedish-based International Geosphere–Biosphere Programme and the French-based Scientific Committee on Problems of the Environment have teamed up to support the International Nitrogen Initiative (INI). This international project is planned as a three-phase effort to assess the state of the knowledge of nitrogen flows and problems, develop region-specific strategies, and put those strategies into place, with regional centers to be established to carry out these goals. The INI will cosponsor the Third International Nitrogen Conference, scheduled for 12–16 October 2004 in Nanjing, China. There, scientists will focus on the problems specific to Asia and examine options for increasing food and energy production while reducing nitrogen pollution. During this meeting, the INI Scientific Advisory Committee will meet to plan one or more regional centers for Asia.
Ultimately, however, the answer is to regulate reactive nitrogen the same as other pollutants, Likens says. In Europe, regulations have helped reduce nitrogen pollution, Galloway says. But the United States—not to mention developing nations—has a long way to go, not just in developing regulations, but in understanding the dynamics of the nitrogen cycle, Galloway says.
He cites the example of federal regulations to reduce nitrogen losses from hog farms. “A lagoon system was mandated to decrease reactive nitrogen–containing waste release into waters. The waste was stored in these big lagoons and then aerated—which released ammonia to the atmosphere—and the sludge was spread onto fields to grow cover crops,” he explains. The system works insofar as it keeps the nitrogen out of the rivers fairly well. “But it just transfers [the nitrogen] to the atmosphere,” Galloway says. “You need to have an integrated management policy.”
We know the global nitrogen system is being disrupted, Galloway says. “What we don’t know is the rate that nitrogen is accumulating. And because reactive nitrogen contributes to many environmental issues of the day, the more you have, the faster the rate of accumulation, and the more you’re going to have an increase in the effects and distribution of the effects.”
“Humans are changing the nitrogen cycle globally faster than any other major biogeochemical cycle—it’s just going through the roof in a hurry,” Townsend says. “The problems with that are remarkably diverse and widespread, and we really need to do something about it. But I think the good news is that there are a lot of ways to envision that we could do something about it without utterly turning socioeconomic systems on their ear.”
Food for a hungry world. Food grown with nitrogen fertilizers feeds an estimated 2 billion people worldwide. Areas including Asia are becoming increasingly dependent on such fertilizers, to the detriment of the environment.
The beauty of storms. Lightning is responsible for fixing a portion of the Earth’s naturally occuring reactive nitrogen, which is important for the soil in areas with few nitrogen-fixing plants.
A good thing gone awry? Nitrogen fertilizers make the difference between an adequate diet and malnutrition for much of the world population, but an excess of reactive nitrogen compounds in the air, water, and soil wreaks havoc on fragile ecosystems.
School of hard NOx. Burning of biomass and combustion of fossil fuels both produce NOx, a significant contributor to the formation of smog and ground-level ozone.
A rising tide. Nitrogen fertilizer runoff contributes to the formation of algal blooms such as this red tide bloom, which extended more than 100 miles along Florida’s Gulf coastline in 2001. Such blooms kill thousands of fish and threaten human health.

Saturation point. Experts warn that nitrogen-saturated soils may not be able to keep the excess from the environment.