Abstract
Background and Objectives
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are a rare diverse group of malignancies, which range from well-differentiated indolent tumors to high-grade aggressive forms. Based on the World Health Organization classification, GEP-NETs are divided into well-differentiated neuroendocrine tumors and poorly differentiated carcinomas. While localized GEP-NETs are primarily treated surgically, non-resectable GEP-NETs have evolved toward targeted therapies, including radioligand therapy. This study describes inpatient resource utilization and inter-regional healthcare mobility for patients with GEP-NETs in Italy, focusing on radioligand therapy.
Methods
We retrieved Italian Hospital Discharge Records (SDO) from 2018 to 2021. Given the absence of specific International Classification of Diseases, Ninth Revision, Clinical Modification codes for GEP-NETs, all potentially related diagnoses were included. Radioligand therapy-related hospitalizations were identified using Diagnosis-Related Group code 409 for radiotherapy, focusing on discharge disciplines of nuclear medicine, radiotherapy, or radiation oncology. We analyzed hospitalization rates by region and regimen and assessed inter-regional mobility using the Attraction and Escape Mobility Indexes.
Results
Over the study period, 4837 radioligand therapy-related GEP-NET hospitalizations were recorded, with 2942 involving the targeted disciplines. Hospitalizations increased by 48.4%, mainly owing to growth in short-stay (0–1 day) discharges (from 37 in 2018 to 228 in 2021), while longer stays (≥ 2 days) rose from 552 to 644. Day hospital accounted for only 0.2% of cases. Regional disparities were prominent, with Emilia-Romagna, Lombardia, and Sicilia managing 88.9% of cases; ten regions recorded no hospitalizations, reflecting a high mobility index (45.8%) and significant inter-regional patient mobility.
Conclusions
The study underscores the need for regulatory adjustments, resource allocation improvements, and healthcare system adaptations to effectively support innovative therapies for GEP-NETs. Addressing these needs is essential to optimize patient outcomes and address regional disparities in Italy’s healthcare system.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-025-01471-6.
Key Points for Decision Makers
| The demand for radioligand therapy in Italy is increasing, due to the growing incidence of gastroenteropancreatic neuroendocrine tumors and recent regulatory changes allowing outpatient treatments. |
| Significant regional disparities in access to radioligand therapy were observed, with only three regions accounting for over 85% of hospital discharges, while ten regions reported no treatments. |
| Defining a dedicated Diagnosis-Related Group code for nuclear medicine procedures is essential to ensure accurate resource allocation and equity in access to innovative treatments, such as radioligand therapy. |
Introduction
Neuroendocrine tumors (NETs) are rare neoplasms that originate from the neuroendocrine system, making them potentially identifiable in any region of the human body. In Italy, every year around 2700 cases of Neuroendocrine neoplasms (NENs) are recorded [1]. Most of the diagnosed cases, approximately two thirds, belong to the category of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) [2]. It is worth noting that Italian epidemiological data are still under analysis, and further insights are expected from the integration of tumor registry data with the national database of the Italian Association for Neuroendocrine Tumors (Itanet) [3].
Gastroenteropancreatic neuroendocrine tumors are a heterogeneous group of malignancies, which range from well-differentiated indolent tumors to high-grade, rapidly progressive tumors [4, 5]. They are categorized based on the World Health Organization classification into two main groups by tumor morphology: well-differentiated NETs and poorly differentiated carcinomas. Neuroendocrine tumors are further subdivided into three grades according to mitotic count and/or Ki-67 index. Grade 1 NETs are characterized by a mitotic count of < 2 per 10 HPF and/or a Ki-67 index of < 3%; Grade 2 NETs have 2–20 mitoses per 10 HPF and/or a Ki-67 index of 3–20%; and Grade 3 NETs demonstrate >20 mitoses per 10 HPF and/or a Ki-67 index of > 20% [6]. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are quite rare, the annual incidence between 2000 and 2012 being 3.56 per 100,000 according to the SEER program [7]. Nevertheless, over the last four decades, the trend of incidence increased steadily, both in the USA [7] and in European countries [8]. The incidence of these tumors is on the rise, thanks to advancements in diagnostic methods in recent years and a deeper understanding of clinical and histopathological aspects [9].
For patients with localized GEP-NETs, curative surgery remains the primary method of treatment, whereas in patients with non-resectable tumors different treatments are considered. While chemotherapy is widely recognized as the first option for treating advanced poorly differentiated carcinomas, several therapeutic options are available for NETs, including somatostatin analogs octreotide and lanreotide, targeted agents (everolimus and sunitinib), and radioligand therapy (RLT) [10, 11].
The introduction of RLT as a treatment option for patients with well-differentiated Grade 1–2 GEP-NETs has significantly expanded the range of available therapies, for those tumors expressing somatostatin receptors, which have progressed despite treatment with somatostatin analogs. This treatment has been proven to be effective both in terms of a substantial tumor reduction, and its ability to delay disease progression [12, 13].
Under the Italian Constitution, responsibility for healthcare is shared by the central government, the 19 regions, and the two autonomous provinces. The shift towards a federal model in the Italian healthcare system had a significant turning point with the approval of the constitutional law in 2001, introducing Title V of the Constitution [14]. This title redefined the allocation of powers between the central State and the regions, granting the latter greater decision-making autonomy in various sectors, including healthcare. Federalism in healthcare has generated several implications, as regions gained more autonomy in planning, organizing, and managing healthcare resources, allowing them to tailor services to the specific needs of the local population. This also meant more responsibility for regions in managing funds allocated to healthcare, in a “quasi-market” context driven by reciprocal competition [15]. In this set-up, the region that owes the debt will assume the responsibility for covering the expenses of medical care for its residents who opt to receive treatment in a different region (the creditor one). A contentious aspect of healthcare federalism is the potential creation of disparities in access to healthcare services and quality of care among different regions. Some regions might have more resources and investment capacity than others, resulting in discrepancies in service provision. The study of the inter-regional flows of hospital patients offers insights into the perceived quality of hospital care and the effectiveness of regional healthcare policies. Mobility serves as a significant indicator, not solely because of its economic implications, but also as a gauge of the fairness of healthcare provision.
In this context, RLT represents a paradigmatic case of a high-complexity treatment that is especially sensitive to healthcare system heterogeneity. Beyond the typical disparities arising from decentralised governance, the delivery of RLT involves specific logistical and infrastructural challenges, such as the need for trained nuclear medicine personnel, specialized equipment, radioisotope supply chains, and strict waste management procedures. These barriers can exacerbate regional inequities, resulting in limited access for patients living in areas lacking the necessary capacity [16]. Consequently, healthcare mobility patterns in RLT are not only a reflection of patient preference or perceived quality, but a direct outcome of systemic constraints in service delivery. Understanding and addressing these constraints is essential for ensuring equitable access to RLT across all regions. The aim of this study was to describe the inpatient resource utilization and the inter-regional healthcare mobility for patients with GEP-NETs in Italy, with a particular focus on RLT.
Materials and Methods
Data Sources
This study relied on data from the Italian Hospital Discharge Records (SDO) for the years 2018–21. The SDO captures all hospital discharges (HDs), including both ordinary inpatient stays and day hospital (DH) admissions, from public and accredited healthcare facilities. Ordinary regimen refers to inpatient hospitalizations where the patient is admitted for at least one overnight stay. It includes any clinical pathway that requires continuous hospital care beyond a single day. Day hospital refers to a hospital admission without an overnight stay. Patients are admitted in the morning for planned diagnostic or therapeutic procedures and discharged the same day. In Italy, DH stays are formally recorded in HD data and treated administratively as inpatient activity, even though they do not involve overnight hospitalization. Accredited hospitals are privately owned institutions that have an agreement with the Italian National Health Service (SSN). They provide healthcare services reimbursed by the public system and must meet specific regulatory and quality standards.
Each record contains, together with a patient-specific anonymous code, patient’s demographic (age, sex, residence) and clinical information (primary and up to five secondary diagnoses and procedures, Diagnosis-Related Group [DRG]). Diagnoses and procedures are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification, Italian version 2007 [17].
Study Population
The study populations were represented by all residents in Italy hospitalized with a diagnosis of GEP-NET. Given the absence of a specific code for GEP-NET, all diagnoses potentially related to GEP-NET were selected (Table 1). A GEP-NET hospitalization was identified if at least one of the codes was present, either as a primary or secondary diagnosis.
Table 1.
ICD9CM codes used to identify HDs related to GEP-NETs
| ICD9CM code | Diagnosis |
|---|---|
| '152.8 | Malignant neoplasm of other specified sites of small intestine |
| '152.9 | Malignant neoplasm of small intestine, unspecified |
| '157.0 | Malignant neoplasm of head of pancreas |
| '157.1 | Malignant neoplasm of body of pancreas |
| '157.2 | Malignant neoplasm of tail of pancreas |
| '157.4 | Malignant neoplasm of islets of langerhans |
| '157.8 | Malignant neoplasm of other specified sites of pancreas |
| '157.9 | Malignant neoplasm of pancreas, part unspecified |
| '194.9 | Malignant neoplasm of endocrine gland, site unspecified |
| '197.7 | Malignant neoplasm of liver, specified as secondary |
| '259.2 | Carcinoid syndrome |
As there is no specific DRG code for RLT, all HDs with DRG 409 “Radiotherapy” were selected. Finally, only HDs with discharge disciplines of nuclear medicine, radiotherapy, or radiation oncology were included in the study.
Analysis
The number of HDs were estimated by the year of observation. Results were stratified by hospital region and by regimen: ordinary with a length of stay ≤ 1 day (ORD 0–1), ordinary with a length of stay ≥ 2 days (ORD ≥ 2), and DH. For ORD ≥ 2, the mean length of stay was reported. The rate of patients hospitalized by region of residence (per 100,000 inhabitants) was estimated across the whole study period.
An analysis of inter-regional healthcare mobility was conducted. For each region, the attraction and escape indexes were calculated as follows. Attraction Mobility Index (AMI) is the number of HDs of non-resident patients out of the total number of region-specific hospitalizations. Escape Mobility Index (EMI) is the proportion between the number of HDs of patients residing in a region and the total number of hospitalizations of residents in that region across the national territory. All analyses were performed using SAS statistical package, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
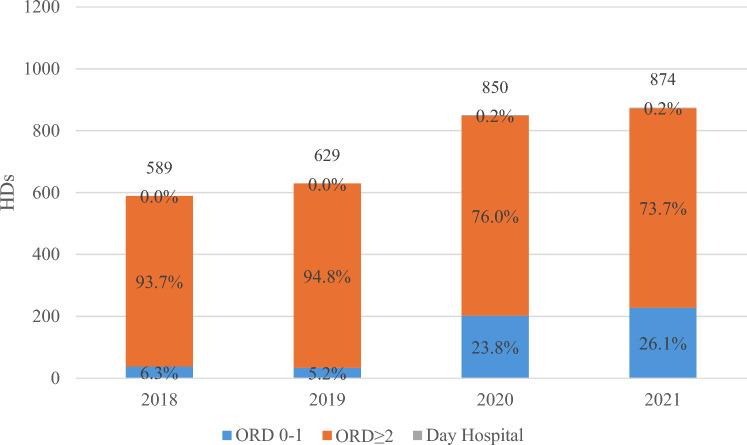
During the study period, 4837 HDs potentially associated with GEP-NETs with radiotherapy (according to DRG 409) were recorded in Italy. Of these, 2942 HDs reported one of the discharge disciplines of interest (nuclear medicine, radiotherapy, or radiation oncology). An increase in the number of HDs was observed from 2018 to 2021 (+ 48.4%), this result mainly depending on the rise in the number of HDs in an ordinary regimen with a length of stay of 0–1 days (37 in 2018, 228 in 2021), while a smaller increase was observed in the number of HDs with a length of stay ≥ 2 days (from 552 to 644, Fig. 1). Daily hospital HDs accounted for, on average, only 0.2% of the total.
Fig. 1.
Hospital discharges (HDs) for gastroenteropancreatic neuroendocrine tumors with radiotherapy by year and regimen. The numbers above the bars represent the total number of hospitalizations per year. The gray section, representing day hospital admissions, is barely visible because of the low number of cases; however, the corresponding percentages are still reported. ORD 0-1 ordinary regimen with a length of stay ≤ 1 day, ORD ≥ 2 ordinary regimen with a length of stay > 1 day
The regional distribution of rates of patients hospitalized (Fig. 2) was quite heterogeneous: a geographical gradient was observed, with higher levels in the north-central area and lower levels in the south, with Sicilia being the only exception. As a matter of fact, the highest rate was observed in Sicilia (9.3 patients per 100,000 inhabitants), followed by Lombardia (7.8), Liguria (7.8), and Emilia-Romagna (6.9). Lowest values were estimated in Basilicata (0.5), Molise (0.6), Campania (0.8), and Puglia (1.9).
Fig. 2.
Rate of patients hospitalized (per 100,000 inhabitants) for gastroenteropancreatic neuroendocrine tumors with radiotherapy in Italy (2018–21). inhab. inhabitants
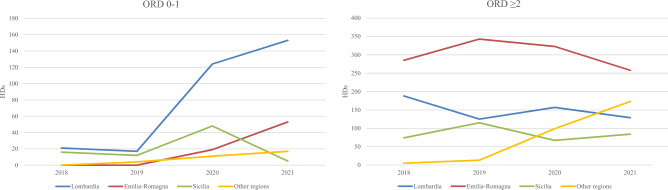
Emilia-Romagna accounted for 43.5% of HDs, followed by Lombardia (31.1%) and Sicilia (14.3%); ten regions did not record any HDs. In Fig. 3, the annual number of HDs by regimen and discharge region are represented. Lombardia and Emilia-Romagna mainly contributed to the positive trend observed for ORD 0–1 HDs (+132 and +53, respectively, 2021 vs 2018), while in Sicily, a pick was observed in 2020, followed by a strong reduction. When considering the other regions, the number of ORD 0–1 HDs went from 0 to 17. Regarding ORD ≥ 2, in Emilia-Romagna, 2019 was the year with the highest number of HDs, followed by a negative trend. In Sicilia and Lombardia, the number of HDs was constant, while a positive trend was observed in the other Italian regions, mainly owing to Toscana (+ 66) and Lazio (+ 46). Distribution of HDs by year, regimen, and disaggregated region of discharge is reported in Table S1 of the Electronic Supplementary Material.
Fig. 3.
Number of hospital discharges (HDs) for gastroenteropancreatic neuroendocrine tumors with radiotherapy by year, discharge region, and regimen. ORD 0-1 ordinary regimen with a length of stay ≤ 1 day, ORD ≥ 2 ordinary regimen with a length of stay >1 day, Other regions Piemonte, Veneto, Liguria, Toscana, Marche, Lazio, Campania, Basilicata
Overall, in the 4-year period considered, in Italy, the mobility index for HDs related to GEP-NET with radiotherapy was equal to 45.8%, i.e., 1347 out of 2942 HDs involved patients admitted in a region different from their residence (Fig. 4). As expected, a reduction in mobility was observed when comparing 2018–19 (54.5%) with 2020–21 (39.6%, results not shown). During the study period, 17 regions reported an EMI higher than 60.0% and, for ten of those, the EMI was equal to 100.0% (no resident had a HD in that region). However, the highest AMI was estimated in Emilia-Romagna, where 992 HDs (AMI 77.4%) were from patients residing elsewhere. Lombardia was the only other region with a high AMI (34.2%, estimated on 914 HDs). Elevated AMI values were also observed in Basilicata (66.7) and Campania (33.3), although these estimates relied on a small number of HDs.
Fig. 4.
Regional distribution of the Attraction Mobility Index (AMI) and Escape Mobility Index (EMI) for gastroenteropancreatic neuroendocrine tumor radiotherapy (2018–21). The numbers in bold on the right represent the total hospitalizations recorded in healthcare facilities located within each region. The numbers on the left indicate the total hospitalizations performed by residents of the respective region
When restricting the analysis to ORD 0–1 HDs, the overall mobility index dropped from 45.8 to 36.4%. A significant reduction in the number of HDs by resident was observed in Piemonte (87 to 6) Emilia-Romagna (1281 to 72), Toscana (96 to 4), and Lazio (75 to 0, Fig. 5).
Fig. 5.
Regional distribution of the Attraction Mobility Index (AMI) and Escape Mobility Index (EMI) for gastroenteropancreatic neuroendocrine tumor radiotherapy in an ordinary regimen with a length of stay ≤ 1 day hospital discharge (2018–21). The numbers in bold on the right represent the total hospitalizations recorded in healthcare facilities located within each region. The numbers on the left indicate the total hospitalizations performed by residents of the respective region
Discussion
The aim of this study was to describe the inpatient resource utilization and the inter-regional healthcare mobility for patients with GEP-NETs in Italy, with a particular focus on RLT. The analysis revealed 2942 HDs potentially associated with GEP-NETs undergoing radiotherapy during the study period. Notably, in the 4-year period considered, there was a marked increase of 48.4% in HDs, primarily driven by a significant rise in ordinary regimen hospitalizations lasting 0–1 days, while a milder increase was recorded for ORD ≥ 2 HDs.
Radioligand therapy with 177Lu-dotatate is currently approved by both the US Food and Drug Administration and the European Medicines Agency for the treatment of unresectable or metastatic, progressive, well-differentiated, Grade 1/2, Somatostatin Recepetor (SSTR)-positive GEP-NETs. In March 2019, the Italian Agency of Drugs (AIFA) approved the use of RLT for the treatment of GEP-NETs [18]. A recent Italian consensus statement from scientific societies (ItaNET, AIOM, SIE, and Italian Association of Nuclear Medicine, Molecular Imaging, and Therapy [AIMN]) strongly recommended for the early integration of RLT for patients with advanced SSTR-positive GEP-NETs that progress after somatostatin analogs [13]. In Italy, since 2020, the overcoming of the requirement of protected stay for medical-nuclear therapies has been established, an undeniable advantage for hospital facilities wishing to organize RLT, especially those lacking protected stay rooms [19]. This is even more crucial when considering that, according to a recent survey, there are 254 nuclear medicine centers in Italy, of which only 47 can guarantee a protected stay, totaling 225 beds (3.7 beds per million inhabitants) [20]. The new regulation also allowed the RLT to be administered on a DH regimen or with hospitalizations with a 0–1 day length of stay [19]. These events could mainly explain the major change in the distribution of the HD regimen before and after 2020.
Regionally, Emilia-Romagna accounted for the highest percentage (43.5%) of HDs related to GEP-NETs with radiotherapy, followed by Lombardia (31.1%) and Sicilia (14.3%). However, the absence of HDs was notable in ten regions, highlighting significant regional disparities, with a regional mobility index equal to 45.8%. High mobility is explained by the reduced number of structures equipped for treatment of GEP-NETs during the study period (2018–21), although the EMI and AMI regional values do not perfectly reflect the regional distribution of facilities. The region with the highest number of nuclear medicine centers was Lazio, with 47 facilities, of which seven had protected hospitalization [21]. Lombardia ranked third (after Campania), with 30 facilities (nine protected), while Emilia-Romagna had only 11 centers (four protected). This could be owing to the fact that not all nuclear medicine centers had implemented guidelines regarding the treatment of GEP-NETs.
The mobility index of 45.8% found in our study is exceptionally high in the context of national healthcare trends. According to recent analyses [22], the national average mobility index for all hospitalizations was 10.2% in 2022, and for high-complexity DRGs it reached 26.5%. These figures reflect an upward trend in mobility, especially considering that the average mobility index during the 2017–19 period was also 8.5%. Importantly, our mobility estimate refers to the 2018–21 period, prior to the 2022 reference year used in the national report. This temporal difference makes our finding even more striking, suggesting that inter-regional mobility for RLT in patients with GEP-NETs was already disproportionately high even before recent increases.
The capacity to deliver RLT is currently insufficient to meet the needs of a growing patient population, especially with the emergence of new indications such as RLT for prostate cancer treatment, or owing to the implementation of new clinical trials that investigate new isotopes (e.g., actinium). The Nuclear Medicine Day Hospital, as well as outpatient therapy, would make it possible, in light of the already approved regulations, to extend therapeutic options even to nuclear medicine centers without protected inpatient facilities. In March 2024, the Italian Association of Medical and Health Physics (AIFM) and the AIMN co-signed a document [23] addressing radioprotection aspects in therapies involving 177Lu currently available. This document outlines scenarios under which these therapies can be administered, in accordance with Legislative Decree 101/2020 and subsequent amendments. For the first time, it references “administration in nuclear medicine with waste collection for at least six hours, followed by patient discharge to their residence [...]” (Scenario 2 of the document) or “[...] potential transfer and admission to a regular ward.” (Scenario 3). This would allow for a more sustainable management economy while expanding therapeutic offerings.
Recent analyses confirm that challenges in ensuring equitable access to RLT are not unique to the Italian healthcare system. According to a report from 2023 [24], disparities in RLT access are widespread across Europe and closely linked to five structural levers: diagnostic capacity, referral processes, availability of authorized centers, regulatory frameworks, and the nuclear medicine workforce. Italy shows the lowest estimated treatment capacity among the countries analyzed, with only five to ten available treatment slots per 100,000 people. In comparison, France reaches 25–30, Germany 55–60, and Spain leads with 75–80 slots per 100,000. Beyond national differences, the report also highlights significant intra-country disparities, particularly in decentralized systems such as Italy and Spain, but also in Germany, where access to RLT is unevenly distributed across regions.
In the coming years, a significant development in nuclear medicine therapy activity is expected owing to the consolidation of traditional therapies and, especially, the rapid advancement of innovative treatments.
In this view, it is noteworthy that on December 2022 the European Medicines Agency approved a new RLT, lutetium (177Lu) vipivotide tetraxetan, for the treatment of progressive prostate-specific membrane, antigen-positive, metastatic castration-resistant prostate cancer. As it is expected that this agent will be reimbursed in Italy in the next few months, the availability of such a novel RLT will dramatically impact on the ability of nuclear medicine departments to meet the needs of the new eligible-to-RTL patients. Compared with the GEP-NET landscape, the population of patients who could potentially benefit from RLT is expected to increase ten-fold [25].
To ensure consistency in treatments and their respective compensations across the entire national territory, an adjustment of the corresponding DRGs is necessary. The absence of a dedicated DRG for nuclear medicine, including procedures related to the administration of RLTs, not only affects the interpretation of hospitalization trends for patients with GEP-NETs but also poses a broader concern for future therapeutic approvals in diseases with larger patient cohorts, such as prostate cancer. This limitation calls for immediate adaptation within healthcare systems to ensure that coding systems adequately capture and encompass new innovative treatments such as RLT. Addressing this issue is paramount to facilitate accurate evaluations of the impact of novel therapies on healthcare resource utilization, patient care, and outcomes across various oncological conditions by developing unbiased strong epidemiological analyses.
Despite the significant contribution of this analysis in describing the hospital care provided to patients with GEP-NETs in Italy, some limitations need to be addressed. First, the analysis is based on administrative data sources which, by their very nature, were not designed for epidemiological purposes but rather for accounting and billing. However, to date, there are no specific registries for tracking and measuring the care and clinical outcomes of patients with GEP-NET, making administrative sources the best available alternative. Second, because of the lack of a specific International Classification of Diseases, Ninth Revision, Clinical Modification code for GEP-NET and a dedicated DRG code for nuclear medicine, there could be potential bias in identifying GEP-NET cases and therapy. Third, it is important to underline that the analysis does not distinguish hospitalizations related to the treatment of patients from those related to clinical trials. Fourth, part of the study period overlaps with the coronavirus disease 2020 pandemic, which may have influenced patient mobility and hospitalization practices. The inter-regional mobility index decreased from 8.55% in 2019 to 7.53% in 2020, then rose again to 8.12% in 2021, indicating a significant but transient reduction [22]. Notably, a national survey by the Italian Association of Radiotherapy and Clinical Oncology reported that most radiotherapy centers resumed nearly full clinical activity as early as mid-2020, during phase II of the pandemic [26]. Therefore, while the pandemic likely had a short-term impact on patient movement and admission duration, its effects were largely mitigated by the second half of 2021. Consequently, the elevated mobility rates observed in our data appear to reflect structural imbalances in RLT service distribution rather than temporary disruptions due to coronavirus disease. Finally, the analysis refers to a period when RLT was available in only a few centers, making it not completely representative of the current situation. A possible future development will be to replicate the study using more recent data and evaluate the impact of the new guidelines on hospital care.
Conclusions
The study underscores the pressing need for regulatory adjustments, resource allocation improvements, and adaptations within healthcare systems to accommodate innovative therapies such as for the treatment of GEP-NETs effectively. Addressing these challenges is vital to ensure equitable access to advanced treatments and enhance the quality of care for patients with GEP-NETs and other oncological conditions in Italy.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement. The study was funded by Novartis Farma S.p.A., on the basis of the transfer of the Advanced Accelerator Applications (Italy) S.r.l. business branch. The funder had no role in the design of the study, the collection, analysis, or interpretation of data, nor in the writing of the manuscript.
Declarations
Conflict of interest
Paolo Sciattella, Matteo Scortichini, and Alfredo Muni have no relevant financial or non-financial interests to disclose. Orazio Caffo has received honoraria as a speaker or advisor for Advanced Accelerator Applications, Astra Zeneca, Astellas, Bayer, Janssen, Ipsen, MSD, Novartis, Pfizer, and Recordati. Marco Maccauro has received advisor honoraria from Advanced Accelerator Applications, Novartis, and Boston Scientific. Francesco Panzuto has received advisor honoraria from Mylan (Viatris), Advanz Pharma, Novartis, Camurus, and Ipsen, and has non-financial interests in the following: AIOM, author of NET guidelines; ENETS, chair of the advisory board; Itanet, chief of the society.
Ethics approval
According to the rules from the Italian Medicines Agency (available from https://www.aifa.gov.it/documents/20142/1654269/Det-Pres-425-2024-Linea_Guida_osservazionali.pdf), retrospective studies using administrative databases do not require ethics committee protocol approval.
Consent to participate
According to the General Authorization for the Processing of Personal Data for Scientific Research Purposes issued by the Italian Privacy Authority on 10 August, 2018 (available from www.garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/9124510), this study was exempt from informed consent.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The SAS code is available from the corresponding author on reasonable request.
Author contributions
Study conception and design: PS, MS, OC, MM, AM, FP; analysis and interpretation of results: PS, MS, OC, MM, AM, FP; draft manuscript preparation: PS, MS, OC, MM, AM, FP. All authors read and approved the final version and agree to be accountable for the work.
References
- 1.Busco S, Buzzoni C, Mallone S, Trama A, Castaing M, Bella F, et al. Italian cancer figures—report 2015: the burden of rare cancers in Italy. Epidemiol Prev. 2016. 10.19191/EP16.1S2.P001.035. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008. 10.1200/JCO.2007.15.4377. [DOI] [PubMed]
- 3.Panzuto F, Partelli S, Campana D, de Braud F, Spada F, Cives M, et al. Epidemiology of gastroenteropancreatic neuroendocrine neoplasms: a review and protocol presentation for bridging tumor registry data with the Italian association for neuroendocrine tumors (Itanet) national database. Endocrine. 2024. 10.1007/s12020-023-03649-4. [DOI] [PMC free article] [PubMed]
- 4.Dasari A, Shen C, Devabhaktuni A, Nighot R, Sorbye H. Survival according to primary tumor location, stage, and treatment patterns in locoregional gastroenteropancreatic high-grade neuroendocrine carcinomas. Oncologist. 2022. 10.1093/oncolo/oyab039. [DOI] [PMC free article] [PubMed]
- 5.Xu Z, Wang L, Dai S, Chen M, Li F, Sun J, et al. Epidemiologic trends of and factors associated with overall survival for patients with gastroenteropancreatic neuroendocrine tumors in the United States. JAMA Netw Open. 2021. 10.1001/jamanetworkopen.2021.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 2022. 10.1007/s12022-022-09708-2. [DOI] [PubMed]
- 7.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017. 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed]
- 8.Leoncini E, Boffetta P, Shafir M, Aleksovska K, Boccia S, Rindi G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine. 2017. 10.1007/s12020-017-1273-x. [DOI] [PMC free article] [PubMed]
- 9.Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological advancements in cancer diagnostics: improvements and limitations. Cancer Rep. 2023. 10.1002/cnr2.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Salvia A, Modica R, Rossi RE, Spada F, Rinzivillo M, Panzuto F, et al. Targeting neuroendocrine tumors with octreotide and lanreotide: key points for clinical practice from NET specialists. Cancer Treat Rev. 2023. 10.1016/j.ctrv.2023.102560. [DOI] [PubMed] [Google Scholar]
- 11.Lamarca A, Bartsch DK, Caplin M, Kos-Kudla B, Kjaer A, Partelli S, et al. European neuroendocrine tumor society (ENETS) 2024 guidance paper for the management of well-differentiated small intestine neuroendocrine tumours. J Neuroendocrinol. 2024;36(9): e13423. [DOI] [PubMed] [Google Scholar]
- 12.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017. 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed]
- 13.Panzuto F, Albertelli M, De Rimini ML, Rizzo FM, Grana CM, Cives M, et al. Radioligand therapy in the therapeutic strategy for patients with gastro-entero-pancreatic neuroendocrine tumors: a consensus statement from the Italian Association for Neuroendocrine Tumors (Itanet), Italian Association of Nuclear Medicine (AIMN), Italian Society of Endocrinology (SIE), Italian Association of Medical Oncology (AIOM). J Endocrinol Invest. 2025;48(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amendments to Title V of Part II of the Constitution, constitutional Law 18/10/2011.
- 15.Grand JL. Quasi-markets and social policy. Econ J. 1991. 10.2307/2234441. [Google Scholar]
- 16.Merkel C, Whicher CH, Bomanji J, Herrmann K, Ćwikła J, Jervis N, et al. Realising the potential of radioligand therapy: policy solutions for the barriers to implementation across Europe. Eur J Nucl Med Mol Imaging. 2020. 10.1007/s00259-020-04745-7. [DOI] [PMC free article] [PubMed]
- 17.Dipartimento Generale Della Programmazione Sanitaria, Direzione Generale del Sistema Informativo. Classificazione Internazionale delle Malattie: ICD-9-CM, Versione Italiana 2007. Italy, 2008.
- 18.Aifa. Determina n. 501/2019: Lutathera. 2019. Available from: http://www.agenziafarmaco.gov.it/it/content/registri-farmaci-sottopos. Accessed 6 Aug 2025.
- 19.D. lgs. 101/2020 Attuazione della direttiva 2013/59/Euratom. Vol. 12, Gazzetta Ufficiale n. 201 del. Available from: https://www.protezionecivile.gov.it/it/normativa/decreto-legislativo-n101-del-31-luglio-2020-0/. Accessed 16 Nov 2023.
- 20.Schillaci O et al. La terapia con radioligandi in oncologia. Libro bianco. RPP Editor, Italy, 2022.
- 21.Maffioli L, Mazzuca N, Bombardieri E. Il libro bianco della medicina nucleare in Italia. AIMN, Italy, 2006.
- 22.Agenas. La mobilità sanitaria in Italia Edizione 2023. Italy, 2023.
- 23.Anna B, Carlo C, Laura A, Sara F, Mahila Esmeralda F, Federica F, et al. Aspetti di radioprotezione nelle terapie con 177 Lu-DOTATATE e 177 Lu-PSMA-617. Associazione Italiana di Fisica Medica e Sanitaria (AIFM) & Associazione Italiana di Medicina Nucleare, Imaging Molecolare e Terapia (AIMN). AIFM, Italy, 2023.
- 24.IQVIA. Succeeding with innovation: the state of radioligand therapy readiness in Europe. 2023. Available from: www.iqviainstitute.org. Accessed 6 Aug 2025.
- 25.Spandonaro F, D’angela D, Polistena B, Bruzzi P, Iacovelli R, Luccarini I, et al. Prevalence of prostate cancer at different clinical stages in Italy: estimated burden of disease based on a modelling study. Biology. 2021. 10.3390/biology10030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jereczek-Fossa BA, Pepa M, Zaffaroni M, Marvaso G, Bruni A, Buglione di Monale e Bastia M, et al. COVID-19 safe and fully operational radiotherapy: an AIRO survey depicting the Italian landscape at the dawn of phase 2. Radiother Oncol. 2021;155:120:122 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.