Abstract
Serological tests with crude or recombinant Leishmania antigens are important tools for the diagnosis of leishmania infection. However, these tests are not markers of active visceral leishmaniasis (VL), since antibodies to these markers are often observed in individuals with subclinical L. chagasi infection and they do not fall shortly after therapy. In this study, levels of immunoglobulin G (IgG) against three recombinant Leishmania antigens (rH2A, KMP11, and the “Q” protein) were evaluated in sera from individuals with subclinical L. chagasi infection and in patients with VL pre- and posttherapy. The sensitivity of the serological test for diagnosis of VL was 100% with all three antigens. The titers of IgG fell significantly after therapy. While most of the individuals with subclinical L. chagasi infection had antibodies to rH2A and the “Q” protein, only 1 out of 15 individuals had antibodies to KMP11. These data indicate that KMP11 may be used to discriminate L. chagasi infection from active VL and may serve as a marker of response to therapy.
Leishmania organisms are obligate intracellular parasites that cause a large clinical spectrum of diseases named leishmaniasis. Visceral leishmaniasis (VL) has been reported in 88 countries, but 90% of VL cases occur in Brazil, India, Sudan, Bangladesh, and Nepal (12). The annual incidence is estimated to be approximately 500,000 cases of VL, and the population at risk is 350 million (1, 5). L. donovani is the causative agent of visceral leishmaniasis in Africa and Asia, L. infantum/chagasi in Mediterranean countries and in the New World (11). VL is a severe debilitating disease that evolves with enlargements of the spleen and liver in addition to lymph nodes. It is characterized by irregular fever, loss of weight, anemia, hepatosplenomegaly, and pancytopenia. However, visceral leishmaniasis occurs in only a small fraction of infected persons, since the majority of the infected individuals have a subclinical form of the infection with self-resolution of the infection (4).
The diagnosis of visceral leishmaniasis is based on Leishmania identification in bone marrow or spleen aspirates. Although identification of leishmania in spleen aspirates is a very sensitive method, this technique cannot be applied to many patients due to the risk of bleeding and the necessity of being performed with hospitalized patients (13). Because of these limitations, immunological indirect methods, such as enzyme-linked immunosorbent assay (ELISA), have been developed to facilitate the diagnosis of VL (12). Thus, serological tests are important tools for diagnosis of VL. However, these tests lack the ability to determine disease activity. For instance, tests using crude Leishmania antigen do not discriminate subclinical infection from VL patients, and antibody titers may remain elevated even years after successful therapy (3). In the present study, we determine the serological levels of immunoglobulin G (IgG) against each of the recombinant antigens (rH2A, KMP11, and the “Q” protein) and compare the findings between subjects with L. chagasi subclinical infection and in patients with VL pre- and posttherapy.
We report that the ELISA tests using the three antigens have a high sensitivity for diagnosis of VL and that the antibody titers fall shortly after therapy. Moreover, we show that while antibodies to rH2A and the “Q” protein were detected in a large group of individuals with subclinical infection, the majority of individuals with L. chagasi infection without disease did not have antibodies to KMP11. These data indicate that serological tests with these recombinant antigens may be helpful as tools to determine therapeutic responses for VL and that the detection of antibodies to KMP11 may help to differentiate subclinical L. chagasi infection from active VL.
MATERIALS AND METHODS
Source of sera.
Sera from 37 VL patients and 30 sera from individuals with subclinical L. chagasi infection were used. The diagnosis of VL was performed by identification of parasites in bone marrow or spleen aspirates. Subclinical infection was defined as a positive serological test for soluble Leishmania antigen and absence of clinical manifestations of VL after 3 years of follow-up. A positive serological test for Leishmania antigens was considered when the optical density (OD) was higher than 0.108, which represents the mean plus three standard deviations of the ODs for healthy subjects unexposed to Leishmania infection. Twenty-nine sera from healthy subjects living in an area to which the disease is not endemic, of ages between 22 and 29 years old, and sera from 15 patients with Chagas' disease were used as a control. Moreover, the sera of 15 patients with VL were obtained pre- and posttherapy. But the amount of sera stored was not enough to perform all the assays. Informed consent was obtained from all patients and controls. The Ethical Committee of Hospital Universitário Professor Edgard Santos and Universidade Federal do Rio Grande do Norte (CEP-UFRN 19/01 and CONEP 4572) assessed and approved the protocol study. In all cases the sera were obtained from blood collected by vein puncture and kept at −20°C until use. The ages of individuals with subclinical L. chagasi infection ranged from 2 to 10 years, those of the VL patients from 2 to 40 years, and those of healthy subjects from 10 to 32 years. The majority of the Brazilian population is a mix of African descendants, Portuguese, and Indians.
Antigens.
KMP11 and rH2A are proteins expressed in L. donovani complex (L. donovani, L. infantum, and L. chagasi). The proteins used in the present work were isolated from L. infantum. The recombinant protein KMP11, a kinetoplastid membrane protein of 11 kDa, was cloned and purified as described previously (7). Leishmania histone H2A is a 14-kDa protein purified as described previously (10). The recombinant antigen “Q” protein is a chimeric protein formed by the genetic fusion of five antigenic determinants from four Leishmania proteins: LiP2a, LiP2b, LiP0, and the histone H2A protein (10). The protein fragments forming the “Q” protein are highly immunogenic during natural L. infantum infections (10).
Serological test: ELISA for antibody detection.
Plate sensitization was done by coating polystyrene, 96-well microtiter plates (Immulon 4; Dynatech Laboratories, Chantilly, VA.) using 100 μl/well of the antigen diluted in carbonate-bicarbonate buffer (0.1 M) (pH 9.6) at a concentration of 1 μg/ml for the three recombinant antigens overnight at 4°C. The plates were washed five times with 200 μl/well of phosphate-buffered saline (PBS) (0.1 M) containing 0.05% Tween 20 (PBS-T). To avoid nonspecific binding, the wells were further blocked with 100 μl of PBS containing 1% Tween 20 for 1 h at room temperature. Afterwards, the sera were diluted 1:50 in PBS-T (100 μl/well) and incubated for 1 h at 37°C. Wells were then washed five times with PBS-T (200 μl/well). After washing, 100 μl/well of anti-human IgG (γ-chain specific) alkaline phosphatase conjugate (Sigma Chemical Co., St. Louis, MO) diluted 1:10,000 with PBS-T was added to each well and further incubated for 1 h at 37°C. After five washes with PBS-T (200 μl/well), the substrate solution, para-nitrophenylphosphate (Sigma Chemical Co.), diluted in carbonate buffer with MgCl2 at a concentration of 1 mg/ml, was added at 50 μl/well. The reaction was developed in the dark at room temperature for 30 min. The absorbance was measured at 405 nm with a Labsystems Multiskan plate reader, and the results are expressed as OD.
Statistical analysis.
The cutoff value for the ELISA for IgG anti-rH2A, anti-KMP11, and Q was defined as the mean OD plus three standard deviations of the values obtained with sera from healthy subjects. The comparison between the ODs from the different groups of patients was performed by Fisher's exact test. Sensitivity and specificity were calculated based on the proportions of true-positive and true-negative values for each test. The Wilcoxon matched-pairs test was used to analyze the data of the VL sera from patients pre- and posttherapy.
RESULTS
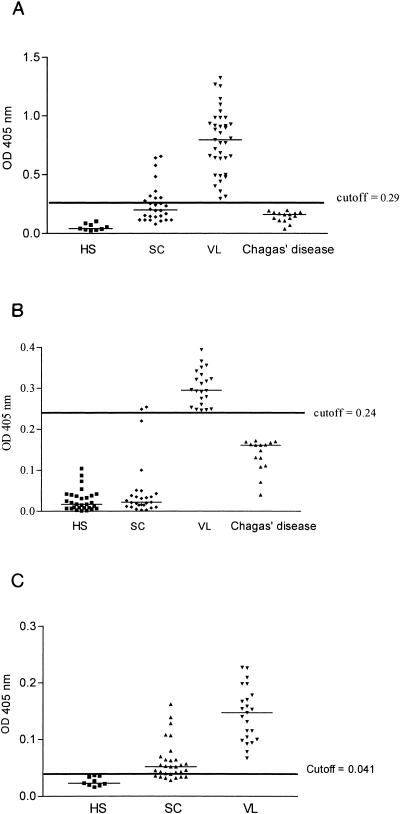
The results of the ELISA test to detect the presence of antibodies against rH2A in VL patients, patients with subclinical (SC) infection, and patients with Chagas' disease are shown in Fig. 1A. The levels of IgG of all VL patients were above the cutoff (sensitivity of 100%). All of the 15 patients with Chagas' disease were negative. Considering the cutoff of 0.29, the specificity was 100% and the predictive positive and negative values were also 100%. The mean ODs of anti-rH2A IgG levels for VL patients, individuals with subclinical L. chagasi infection, and patients with Chagas' disease were 0.78 ± 0.27, 0.25 ± 0.16, and 0.16 ± 0.09, respectively. The test was not able to discriminate subclinical infection from VL, since antibodies to rH2A were detected in 33% of the individuals with subclinical infection.
FIG. 1.
A. Levels of anti-rH2A IgG detected in the sera of 9 healthy subjects (HS), 28 individuals with subclinical L. chagasi infection (SC), 37 visceral leishmaniasis patients (VL), and 15 patients with Chagas' disease. The cutoff value was defined as the mean optical density plus three standard deviations for the values obtained from sera of healthy subjects plus the values for the patients with Chagas' disease (control group). In each group, the median line appears. B. Levels of anti-KMP11 IgG detected in the sera of 29 healthy subjects, 22 patients with visceral leishmaniasis, 27 individuals with subclinical L. chagasi infection, and 15 patients with Chagas' disease. The cutoff value was defined as the mean optical density plus three standard deviations for the values obtained from sera of healthy subjects plus the values for the patients with Chagas' disease (control group). In each group, the median line appears. C. Levels of anti-“Q” protein IgG detected in sera of 9 healthy subjects, 30 individuals with subclinical L. chagasi infection, and 23 patients with visceral leishmaniasis. The cutoff value was defined as the mean optical density plus three standard deviations for the values obtained from sera of healthy subjects. In each group, the median line appears.
The anti-KMP11 antibodies present in the sera of VL patients, subjects with SC infection, and patients with Chagas' disease are shown in Fig. 1B. The IgG levels of all VL patients were above the cutoff (OD of 0.24). The test was positive for two of the SC individuals and was negative for all patients with Chagas' disease. The sensitivity of the test for diagnosis of VL was 100%, and the predictive positive and negative values were also 100% (Fig. 1B). The mean OD of anti-KMP11 IgG levels in VL patients (0.30 ± 0.04) was higher than that observed for individuals with subclinical L. chagasi infection (0.05 ± 0.07 (P < 0.05) and in patients with Chagas' disease (0.16 ± 0.09).
The levels of IgG against the recombinant “Q” protein from VL patients, individuals with SC L. chagasi infection, and healthy subjects (HS) are shown in Fig. 1C. The IgG levels for all VL patients were above the cutoff (OD of 0.041). The mean ODs of anti-“Q” IgG for the sera of VL patients, individuals with SC L. chagasi infection, patients with Chagas' disease, and healthy subjects were 0.15 ± 0.03, 0.14 ± 0.04, 0.06 ± 0.01, and 0.14 ± 0.08, respectively. The test was positive for 13 of 30 SC individuals. The sensitivity of the test for diagnosis of VL was 100%, and the specificity was 43%. The predictive positive and negative values were, respectively, 100% (Fig. 1C). As expected, all subclinical sera for rH2A were positive for the “Q” protein. However, five sera positive for the “Q” protein were negative for rH2A.
The decreases in the levels of IgG anti-rH2A, anti-KMP11, and anti-“Q” protein detected in the sera of patients with VL pre- and posttreatment are shown in the Fig. 2A, B, and C, respectively. These sera were obtained 30 to 60 days after antimony therapy. All VL patients were successfully cured. As shown in the figure, the OD of the IgG antibody against each of these antigens decreased significantly after treatment (P < 0.05).
FIG. 2.
A. Levels of anti-rH2A IgG detected in sera of 15 patients with visceral leishmaniasis pre- and posttreatment (Pre and Post). The P value was calculated with the Wilcoxon matched-pairs test, and P values of <0.05 were considered significant. B. Levels of anti-KMP11 IgG detected in sera from 15 patients with visceral leishmaniasis pre- and posttreatment. The P value was calculated with the Wilcoxon matched-pairs test, and P values of <0.05 were considered significant. C. Levels of anti-“Q” protein IgG detected in sera of eight patients with visceral leishmaniasis pre- and posttreatment. The P value was calculated with the Wilcoxon matched-pairs test, and P values of <0.05 were considered significant.
DISCUSSION
Previous reports from areas of L. chagasi transmission have shown that less than 15% of individuals infected with L. chagasi develop the classical VL (6, 9). The majority of the infected individuals will develop a subclinical form of L. chagasi infection that may remain completely asymptomatic or may progress to VL. In the present study, individuals infected with L. chagasi were followed for up to 3 years after sera conversion. Since none of them developed symptoms of VL, they were considered to have an asymptomatic form of the L. chagasi infection (9). Tests with crude Leishmania antigen are widely used for diagnosis of VL but did not discriminate between L. chagasi infection and VL (9). Additionally, the tests with crude Leishmania antigen cannot be used as markers of cured VL, since antibody may persist for a long period of time (3, 5). For all of the antigens evaluated in the present study, the sensitivity for the diagnosis of VL using an ELISA test was 100%. Moreover, a large proportion of SC individuals had a positive test result for rH2A and the chimeric protein but not for KMP11. A previous study which evaluated the reactivity against rK39 suggested that this antigen could discriminate L. chagasi infection from VL (2). In that study, the individuals with a SC infection were selected based on a positive leishmania skin test. We have shown previously that antileishmanial antibodies decrease parallel to conversion of the skin test (8). Therefore, it cannot be ruled out that the discrimination between Leishmania infection and VL observed in that study was due to the absence of or to a very low titer of antibody to Leishmania antigens. In the present study, the individuals with subclinical L. chagasi infection were selected based on a positive serology. The documentation that only 2 of 27 L. chagasi-infected individuals without disease had a positive serological test when the KMP11 was used indicate that this test is helpful for the discrimination of subclinical infection from classical VL.
Antimony continues to be the first drug of choice for treatment of VL in Brazil. Treatment with this drug has a high rate of cure, but VL patients recently discharged from the hospital still present substantial clinical and biochemical features associated with the disease. Enlargement of the spleen and liver, anemia, leukopenia, and hyperglobulinemia may persist for weeks after therapy, and the conversion to a positive skin test usually occurs after 6 months to a year after successful treatment. Therefore, it is important to have a test that evaluates the response to the therapy. Previous studies have shown that serological test for Leishmania may remain positive for years (3, 4). Here we show that a significant fall in levels of antibody to rH2A, KMP11, and the “Q” protein occurs shortly after therapy. These data support the idea, therefore, that the analysis of the reactivity against these proteins during antimony treatment may be used as a marker of a therapeutic response and recovery of VL.
Acknowledgments
This work was supported by NIH grant AI-30639 and the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) and Brazilian National Research Council (CNPq). Edgar M. Carvalho is a senior investigator of CNPq.
REFERENCES
- 1.Boelaert, M., B. Criel, J. Leeuwenburg, W. Van Damme, D. Le Ray, and P. Van der Stuyft. 2000. Visceral leishmaniasis control: a public health perspective. Trans. R. Soc. Trop. Med. Hyg. 94:465-471. [DOI] [PubMed] [Google Scholar]
- 2.Braz, R. F., E. T. Nascimento, D. R. Martins, M. E. Wilson, R. D. Pearson, S. G. Reed, and S. M. Jeronimo. 2002. The sensitivity and specificity of Leishmania chagasi recombinant K39 antigen in the diagnosis of American visceral leishmaniasis and in differentiating active from subclinical infection. Am. J. Trop. Med. Hyg. 67:344-348. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho, E. M., R. Badaro, S. G. Reed, T. C. Jones, and W. D. Johnson, Jr. 1985. Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J. Clin. Investig. 76:2066-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho, E. M., A. Barral, D. Pedral-Sampaio, M. Barral-Netto, R. Badaro, H. Rocha, and W. D. Johnson, Jr. 1992. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J. Infect. Dis. 165:535-540. [DOI] [PubMed] [Google Scholar]
- 5.Desjeux, P. 1996. Leishmaniasis. Public health aspects and control. Clin. Dermatol. 14:417-423. [DOI] [PubMed] [Google Scholar]
- 6.Evans, T. G., M. J. Teixeira, I. T. McAuliffe, I. Vasconcelos, A. W. Vasconcelos, A. Sousa Ade, J. W. Lima, and R. D. Pearson. 1992. Epidemiology of visceral leishmaniasis in northeast Brazil. J. Infect. Dis. 166:1124-1132. [DOI] [PubMed] [Google Scholar]
- 7.Fuertes, M. A., J. M. Perez, M. Soto, M. C. Lopez, and C. Alonso. 2001. Calcium-induced conformational changes in Leishmania infantum kinetoplastid membrane protein-11. J. Biol. Inorg. Chem. 6:107-117. [DOI] [PubMed] [Google Scholar]
- 8.Miles, S. A., S. M. Conrad, R. G. Alves, S. M. Jeronimo, and D. M. Mosser. 2005. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 201:747-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed, S. G. 1996. Diagnosis of leishmaniasis. Clin. Dermatol. 14:471-478. [DOI] [PubMed] [Google Scholar]
- 10.Soto, M., J. M. Requena, L. Quijada, and C. Alonso. 1998. Multicomponent chimeric antigen for serodiagnosis of canine visceral leishmaniasis. J. Clin. Microbiol. 36:58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 1990. Control of the leishmaniasis, p. 1-158. Technical report series no. 793. World Health Organization, Geneva, Switzerland. [PubMed]
- 12.World Health Organization. 2002. Strategic direction: leishmaniasis. World Health Organization, Geneva, Switzerland.
- 13.Zijlstra, E. E. 1992. The treatment of kala-azar: old and new options. Trop. Geogr. Med. 44:288. [PubMed] [Google Scholar]