Abstract
Dendritic cells (DCs) are professional antigen-presenting cells that can prime T cells and polarize the cellular immune response. Because Th1-type immune responses have been connected to success in combating viral infection, a promising therapeutic application of DCs would be their differentiation in vitro and injection back into the host to boost an immune response in infected animals. This study was aimed both at developing a protocol to cultivate feline DCs in the absence of exogenous proteins for their use in vivo and at investigating what might be the most appropriate stimulus to induce their maturation in vitro and finding correlates of maturation. We generated DCs from peripheral blood monocytes in the presence of feline interleukin-4 and granulocyte-macrophage colony stimulating factor, and after 5 days their maturation was induced with either lipopolysaccharide, human recombinant tumor necrosis factor alpha, poly(I:C), or activated feline platelets. After 48 h, their CD14, CD1a, major histocompatibility complex class II, and B7.1 surface expression was analyzed in parallel with their ability to uptake antigen or prime a mixed leukocyte reaction. The results presented show that feline DCs cultured in autologous plasma differentiate and are able to mature in the presence of stimuli similar to the ones currently used for other species. The present work sets the grounds for future use of DCs obtained by the protocol described for in vivo vaccination and immunotherapy of feline immunodeficiency virus-infected cats.
Dendritic cells (DCs) are professional antigen-presenting cells that can initiate an immune response by priming naïve lymphocytes and polarizing them to give rise to Th1 or Th2 type responses (21). In humans and other species, DC progenitor cells, such as CD14+ monocytes, are present in peripheral blood and CD14+ and/or adherent peripheral blood mononuclear cells (PBMCs) can be induced to differentiate in culture into monocyte-derived DCs in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) (1, 16, 29). Under these conditions, monocytes develop into immature DCs (iDCs). Typical features characterize iDCs: they are nonadherent and present many long processes, and upon staining with specific antibodies, human DCs are CD14− while they express markers such as CD1a, CD86, and DC-SIGN (8, 17). As concerns their function, iDCs are particularly efficient at uptaking antigen by different mechanisms (19). The full potential of DCs as antigen-presenting cells is achieved under a wide variety of stimuli in vivo, mostly of the inflammatory type, that induce iDCs to mature. Mature DCs (mDCs) are less capable of uptaking antigen but they express much larger amounts of major histocompatibility complex (MHC) and costimulatory molecules on their surface, such as the specific marker CD83 (28) and migrate more efficiently to lymph nodes (13).
A number of stimuli have been reported in the literature that drive iDCs to mature. Microbial molecules, such as lipopolysaccharide (LPS) or double-stranded RNA, are potent inducers of DC maturation (3, 26). On the other hand, inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), are currently used to induce maturation of DCs for clinical use (10). Other stimuli, such as CD40 triggering, for example by activated platelets that normally express CD40L (7), have also been used. All the stimuli mentioned act through different receptors and are likely to bring about distinct developmental events.
The fact that DCs can differentiate in vitro and that they can present antigen very efficiently make them ideal live adjuvant/immunostimulators for vaccination and/or therapy. Immunotherapy using ex vivo-generated DCs has recently been shown to enhance protective immunity against simian immunodeficiency virus infection in macaques and in human immunodeficiency virus-infected humans (9, 10) and clinical studies have been performed on humans in which tumor antigen-loaded DCs were used to boost immune responses against malignant tumors (15).
Several protocols have been established to prepare human DCs suitable for in vivo use (5). On the other hand, very few studies have been performed so far on the characterization and culture conditions for feline DCs (1, 4). Feline immunodeficiency virus infection in cats has long been studied as a model for human AIDS, but very little is known on the role of DCs in this particular context. Therefore, the present study was undertaken in order to (i) establish the conditions to culture sizeable numbers of “clinical grade” feline DCs, (ii) assess what stimulus would be most suitable to induce efficient maturation of monocyte-derived DCs, and (iii) select parameters predicting mDC ability to better induce an immune response with the aim of establishing a protocol to use DCs in vivo in vaccine/immunotherapy experiments.
To make sure that feline cells would make contact with as few foreign antigens as possible during in vitro culture, the use of fetal calf serum (FCS) was avoided by using autologous plasma and only carrier-free feline recombinant cytokines were used when available. The method described allows culture and differentiation of CD14+ cells present in PBMCs obtained from heparinized peripheral blood and their differentiation into iDCs. We have also tested different factors known to induce human DC maturation to assess which would be the most appropriate to induce feline DC maturation in terms of capacity to promote alloreactivity.
MATERIALS AND METHODS
Media and reagents.
The cell culture medium used was RPMI 1640 supplemented with 3% autologous feline plasma, unless otherwise stated, 2 mM l-glutamine, 1% nonessential amino acids, and 50 μg/ml gentamicin. Feline serum was obtained as a pool from specific-pathogen-free (SPF) cats in our animal facility and was used after heat inactivation. Recombinant feline IL-4 and recombinant feline GM-CSF were purchased at R&D Systems (Minneapolis, MN). Monoclonal antibodies used in this study were anti-CD14-phycoerythrin (TUK4), which cross-reacts with feline CD14 (1), and its negative isotype-matched control (Serotec, Raleigh, NC), anti-feline CD1a (FE1.5F4) and MHC class II (42.3), provided by Peter F. Moore, University of California, Davis. Anti-feline B7.1 (B7.1.66) was a kind gift from Wayne Tompkins, North Carolina State University (25). The negative control for all unlabeled immunoglobulin G1 (IgG1) isotype antibodies was monoclonal antibody L8D8 (6). All unlabeled monoclonal antibodies were mouse IgG1. Fluorescein-labeled goat anti-mouse IgG (Sigma) was used as a secondary reagent.
Maturation stimuli.
LPS from Escherichia coli 0127:B8 (Sigma Chemical Co., St. Louis, MO) was used at 50 ng/ml. Poly(I:C) was obtained from Amersham Biosciences (Uppsala, Sweden) and diluted in saline following the manufacturer’s instructions. Recombinant human TNF-α was obtained from R&D and tested at 100 ng/ml. Recombinant human alpha interferon (IFN-α; Roferon-A) was purchased from Roche (Monza, Italy) while natural human IFN-α (Cilferon-A) was purchased from Janssen (Beerse, Belgium); they were used at 1,000 U/ml. Platelets were isolated from EDTA-treated whole blood as follows: 10 ml blood was centrifuged for 15 min at 160 × g. Then, 4 ml turbid platelet-rich plasma was removed from the upper layer. Platelets were obtained from platelet-rich plasma by centrifuging for 15 min at 600 × g. The platelet pellet was resuspended in serum-free RPMI 1640, and platelets were counted. They were then activated for 2 min with 20 μM ADP (Calbiochem, San Diego, CA), and brought to 15 ml in saline-1% paraformaldehyde. The suspension was left 90 min at room temperature; then it was washed twice in RPMI 1640. Fixed activated platelets were resuspended at 109/ml and stored in aliquots at −80°C.
Animals and culture conditions.
SPF female cats were bought from IFFA Credo (L'Arbresle, France). Heparinized jugular venous blood was obtained from lightly anesthetized 12- to 24-month-old cats kept in our climatized animal facility under conditions required by the European Community Law. A maximum of 35 ml was drawn at any one time. PBMCs were obtained by gradient centrifugation over a Ficoll-Paque layer for 30 min at 550 × g. The cells isolated were washed in saline, counted and resuspended in medium at 3 × 106 cells/ml. PBMC yield varied between 70 and 200 × 106. Three-milliter aliquots were distributed in six-well plates, and then autologous plasma was added. Fresh PBMCs at 105 were stained for CD14 expression and analyzed by fluorescence-activated cell sorter (FACS) to calculate CD14+ cell percentage of cells with a forward scatter (FSC) of >200.
After 24 h, nonadherent cells were removed while adhering cells were washed delicately twice with warm medium and 1 ml medium containing 3% autologous plasma, recombinant feline IL-4, 10 ng/ml, and recombinant feline GM-CSF, 50 ng/ml, was added. Every other day, the same quantities of IL-4 and GM-CSF were added in 100 μl of medium. Forty-eight hours before analysis, fresh cytokines and maturation agents (see above) were added. Cells were cultured for a total of 7 days, unless otherwise stated. For light microscopy pictures, cells were cytospun onto glass slides and stained with Giemsa stain (Sigma).
CD14+ cell purification.
CD14+ cells were obtained from freshly isolated PBMCs by magnetic activated cell sorting (MACS) following the manufacturers' instructions: 20 × 106 PBMCs were incubated for 15 min with 40 μl of anti-human CD14 microbeads (Miltenyi Biotec, Cologne, Germany). These beads cross-react with feline CD14 (27). After washing, cells were loaded onto a mass spectrometry column (Miltenyi Biotec). Around 1 × 106 to 3 × 106 CD14+ cells were normally obtained, while purity ranged between 70 and 80%. A total of 5 × 105 CD14+ cells were cultured in 1 ml of cytokine-containing medium. From here on, cells were treated as the ones obtained by adherence.
FACS analysis.
The day of analysis, cells were vigorously harvested so that all cells were removed from wells. Staining was carried out in RPMI, 0.2% bovine serum albumin, and 0.1% Na azide on ice for 30 min. Isotype-matched control antibodies were used as negative controls. A fluoresceinated goat anti-mouse IgG polyclonal antiserum (Sigma) was used as a secondary antibody where needed. Before analysis, cells were fixed in phosphate-buffered saline (PBS) and 1% paraformaldehyde for 20 min on ice. Alternatively, to exclude dead cells, cells were resuspended in PBS, 0.2% bovine serum albumin, and 0.1% Na azide, 300 μl, and 2 μl of 100 μg/ml propidium iodide was added.
All flow cytometry data were acquired with a CellQuest software (Becton Dickinson, San Diego, CA) and analyzed by WinMDI. Dead cells and lymphocytes were excluded by light scatter properties and propidium iodide staining.
Phagocytosis.
At the time of analysis, cells resuspended in RPMI 1640 were supplemented with 10% FCS and with 200 μg/ml FITC-labeled dextran, molecular weight 40,000 (FITC-DX, Molecular Probes), or 100 μg/ml Alexa Fluor 488 hydrazide (Alexa; Invitrogen, Milan, Italy) in duplicate. One vial was incubated for 45 min, unless otherwise stated, at 37°C, 5% CO2, while the other was left on ice. Cells were then washed, fixed and analyzed by flow cytometry as described above.
Mixed leucocyte reaction.
MΦs, iDCs, and mDCs were tested for their ability to stimulate a response by allogeneic PBMCs. Cells grown as described above were harvested at day 7 of culture and 1,000, 3,000, or 10,000 were added to 105 allogeneic PBMCs in 96-well plates in triplicate. Cells were cultured for 4 days in RPMI 1640 and 10% human serum, after which proliferation was assessed by adding 1 μCi [methyl-3H]thymidine (Amersham Biosciences) per well 18 h before harvesting the cells on filters and counting radioactivity in a beta-scintillation counter.
Statistical analyses.
Statistical analyses were performed using Student's t test.
RESULTS
Generation of feline DCs from cultured adherent PBMCs.
Our initial effort was devoted to setting up conditions to grow feline DCs with the final aim of injecting them back into cats. Therefore, the use of FCS was avoided and only carrier-free feline recombinant cytokines were used, when available, to prevent DCs from getting loaded with xenogeneic antigens.
Feline PBMCs were obtained as described in Materials and Methods. On average, from 2 × 106 to 6 × 106 PBMCs/ml of blood were obtained, of which 4 to 8% were CD14+. DC culture was attempted by two different methods: (i) culture of purified CD14+ monocytes and (ii) culture of cells adhering to plastic after 24 h. When 3% autologous plasma was used, only adherent monocytes proved to differentiate in culture, while purified CD14+ cells from the same cats gradually died out over a period of 6 days in three different experiments. Purified CD14+ monocytes did not survive even when complement in autologous plasma was inactivated by incubating at 56°C for 30 min. Curiously, they did grow in RPMI medium containing 10% FCS and in the latter conditions they differentiated into DC-looking cells (data not shown). DCs were, therefore, obtained by adherence of monocytes to plastic after a 24-h incubation at 37°C, 5% CO2 and differentiation in IL-4- and GM-CSF-containing medium for at least 5 days.
Different serum supplements were tested: adherent PBMCs were grown in the presence of 10% FCS; 3%, 5%, and 10% autologous heparinized plasma; and 3%, 5%, and 10% heat-inactivated cat serum. The best results in terms of DC aspect and number were obtained with 3% autologous plasma. Interestingly, autologous plasma obtained from blood containing EDTA instead of heparin as an anticlotting agent turned out to be toxic to cells.
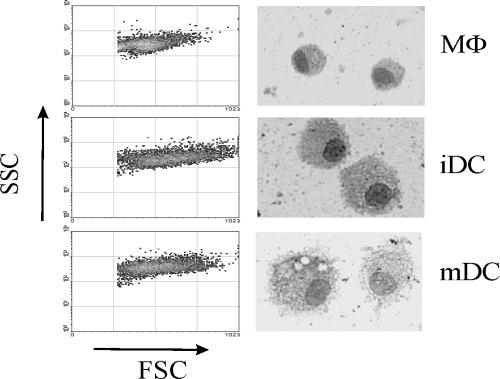
Plastic adherence was initially carried out for 3 h, but adhering cells could still be found in the population removed from the wells after such a period; therefore, adherence time was prolonged to 24 h. Addition of medium containing 10 ng/ml feline IL-4 and 50 ng/ml feline GM-CSF to adherent cells caused them to differentiate into a uniform population, as seen by their light scatter properties, already after 24 h. Typical DC morphology could be seen by light microscopy and was maintained for at least 7 days (Fig. 1, right): these cells were larger in size than MΦs, as also confirmed by their FSC by side scatter (SSC) profile (Fig. 1, left). In these conditions, T cells were not found to grow significantly, as opposed to what happened when human PBMCs were grown the same way in our laboratory and in others (data not shown) (17). Contamination by normal platelets did not seem to influence DC differentiation: experiments where care was taken to remove platelets yielded comparable results in terms of DC phenotype and numbers; therefore no special step was performed to remove contaminating platelets. Purity could not be exactly determined due to the absence of specific DC markers; however, in initial experiments, DC populations were also stained with feline anti-CD4 and anti-CD8 and less than 2% positive cells were detected (data not shown).
FIG. 1.
Different morphology of MΦs, iDCs and mDCs. Adherent PBMCs were cultured for 7 days with (iDC) or without (MΦ) IL-4 and GM-CSF. To obtain mDCs, 50 ng/ml of LPS was added to iDCs 48 h before analysis. Light scatter properties (left) and microscopic appearance of cytocentrifuged MΦs, iDCs, and mDCs stained with Giemsa and photographed at ×356 (right) are shown.
In many experiments, cultures of 24-h adherent cells in the absence of cytokines were also set up to obtain MΦs. In these conditions, we obtained both adherent and nonadherent cells, as previously described (1). MΦs were generally smaller than DCs and less granular (Fig. 1). These results show that a homogeneous population of cells with DC morphology differentiated from adherent feline PBMCs in the presence of GM-CSF and IL-4 under the culture conditions selected. These cells are referred to as iDCs.
Maturation of feline DCs has never been reported to our knowledge. In order to assess whether feline monocyte-derived DCs underwent maturation in a similar fashion to primate and murine DCs, we tested whether the addition of LPS to iDC cultures brought about any change. LPS, which interacts with CD14, has been shown to be a very strong maturation stimulus (20); therefore iDCs were supplemented with 50 ng/ml LPS 2 days before analysis. The addition of LPS caused the cells to become highly vesiculated, as shown by Fig. 1: their appearance at the microscope was different from iDCs (rounder and smaller) and their SSC was increased. These cells were referred to as mDCs.
Characterization of feline DCs by flow cytometry.
To characterize feline DCs, iDCs and LPS-treated mDCs were analyzed for their surface expression of distinctive marker molecules. Because there are no antibodies exclusively specific for feline DCs, to monitor DC maturation we evaluated changes in MHC class II, CD1a, B7.1 and CD14 expression trying to correlate the level of these molecules on the DC surface with their capability to uptake antigen and/or induce alloreactive T cells to proliferate, a distinctive endowment of mDCs. To test the off chance of cross-species reactivity of commercial antibodies against feline and human DC-SIGN and CD83, we did try anti-human CD83 and DC-SIGN antibodies (see Materials and Methods) (8, 28) on feline mDCs and iDCs but no reactivity was detected (data not shown).
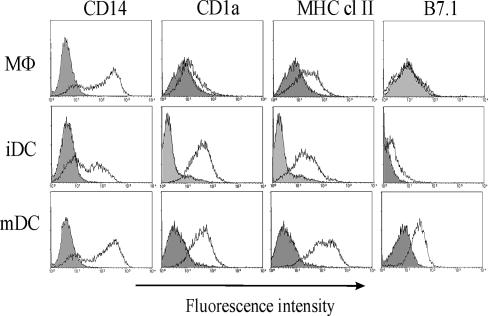
A homogeneous population of cells with high FSC (Fig. 1) was analyzed for fluorescence in at least five different experiments. Based on results obtained with human DCs by others, CD14 was expected to decrease on the surface of feline DCs in general, CD1a and MHC class II were expected to increase on DCs, and B7.1 was expected to be highly expressed on DCs. Figure 2 shows typical results obtained from one representative cat: as far as CD14 expression is concerned, feline DCs followed a pattern that seemed different from the one described for human DCs: MΦs were practically all positive and extremely high in CD14 expression, while iDCs were either negative for CD14 or on average three to five times lower in CD14 expression than MΦs. This is quite different from what was observed by Bienzle et al., who detected an increase in CD14 expression on iDCs compared to MΦs (1). Treatment with LPS for 48 h increased both the percentage of CD14+ cells compared to iDCs and their level of CD14 expression to values comparable to the ones seen for MΦs. When MΦs, iDCs, and mDCs were compared for CD1a expression, DCs in general expressed higher levels of such a marker on their surface: as depicted in Fig. 2, iDCs were roughly five times as bright for CD1a compared to ΜΦs. Even if expression of CD1a could be detected on feline MΦs, as reported by others (1), it was very low (Fig. 2). No significant difference could be detected between CD1a expression on iDCs and mDCs. Maturation, therefore, seemed to have no effect on CD1a expression. On the other hand, MHC class II expression increased from MΦs to iDCs to mDCs, and B7.1 seemed to be very poorly expressed on MΦs, but it was present on iDCs and even more on mDCs.
FIG. 2.
Comparison of surface phenotype of MΦs, iDCs and mDCs. Adherent PBMCs were cultured as described in the legend to Fig. 1. Histogram plots of MΦs, iDCs and mDCs stained with CD14, CD1a, MHC class II or B7.1 (open histograms) were overlaid with the respective isotype-matched negative controls (shaded histograms). Data are representative of at least four experiments.
To sum up, in our feline model, MΦs were mostly CD14hi, CD1alo, MHClo, and B7.1−; iDCs were CD14lo or CD14−, CD1ahi, MHC+, and B7.1lo; and LPS-treated mDCs were CD14hi, CD1ahi, MHChi and B7.1hi.
Phagocytic ability of feline DCs.
DCs have been shown to be able to concentrate antigen in the MHC class II compartment by at least two mechanisms: macropinocytosis and mannose receptor-mediated uptake (20) This capacity is typically marked in iDCs while it is downregulated after maturation, probably because mDCs have mostly switched from uptaking Ag to presenting it to T cells.
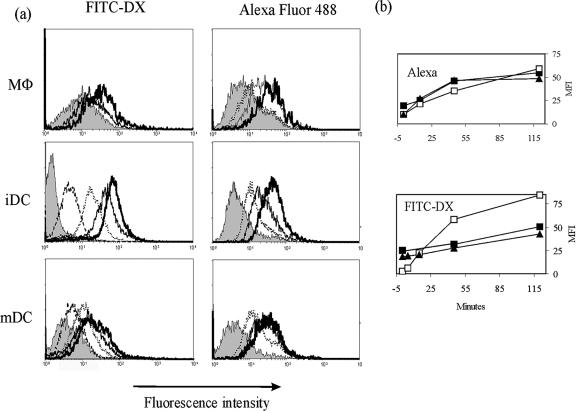
To evaluate the phagocytic ability of feline DCs, we compared antigen uptake by immature and LPS-matured feline DCs in at least three different experiments by using FITC-DX, which is taken up by the mannose receptor (20), and Alexa, a soluble fluorescent substance behaving as a fluid phase marker taken up by pinocytosis (1). Cells were incubated with FITC-DX or Alexa either for 120 min on ice or for different times at 37°C and then fixed and analyzed by FACS.
Figure 3a shows typical results obtained from a representative experiment: iDCs accumulated both FITC-DX and Alexa in a time-dependent fashion; however, accumulation of FITC-DX was most efficiently performed by iDCs within the first hour of incubation (Fig. 3b), while they tended to slow down after such time. After 120 min of incubation at 37°C, iDCs were roughly 80 times brighter than after incubation for the same time at 0°C (Fig. 3b). Most iDCs tended to accumulate FITC-DX at the same rate, which led to narrow peaks in the FACS histograms. MΦs and mDCs were more heterogeneous in this respect and tended to differ quite a lot in their FITC-DX uptake, as shown by the broad fluorescence range in histograms (Fig. 3a). Overall, LPS-treated mDCs seemed as efficient as MΦs in taking up FITC-DX, as can be seen in Fig. 3b: both types of cells accumulate FITC-DX linearly in time over 2 h, differently from iDCs. After a 2-h incubation at 37°C, both populations were roughly seven times more fluorescent than background.
FIG. 3.
Kinetics of uptake of fluorescent molecules by feline MΦs, iDCs, and mDCs. (a) MΦs, iDCs and LPS-treated mDCs, obtained as described in Fig. 1, were analyzed by FACS after incubation with 200 μg/ml FITC-DX or 100 μg/ml Alexa for 120 min on ice (filled histograms) or at 37°C for 5 (dashed lines, iDC and mDC plus FITC-DX only), 15 (dotted lines), 45 (solid lines), and 120 (bold lines) min. (b) Mean fluorescence intensity (MFI) of each population after each time stated was subtracted of MFI after 120 min at 0°C and plotted against time of incubation. MΦs (solid squares), iDCs (empty squares), and mDCs (triangles) are shown. One representative experiment of three is shown.
Accumulation of Alexa, on the other hand, tended to be more similar in MΦs, iDCs, and mDCs, although iDCs are still slightly more efficient (Fig. 3b).
Thus, FITC-DX uptake allows good distinction between iDCs and mDCs/MΦs: LPS induced iDCs to downregulate their ability to uptake antigen via the mannose receptor. This suggests that feline DCs mature similarly to those of other species following LPS administration.
Primary allo-MLR by feline mDCs.
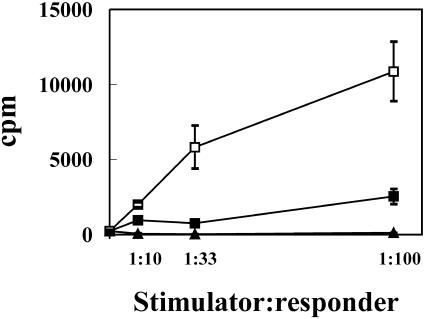
DCs are known to be the best antigen-presenting cells in terms of their capacity to prime naïve T cells. This feature is particularly pronounced in mDCs, seemingly because molecules such as MHC class II and costimulatory molecules such as B7 and others are upregulated (21, 24). Therefore, to confirm that LPS induced feline DC maturation and to assess the stimulatory capacity of feline DCs, we compared the ability of MΦs, iDCs and LPS-treated mDCs to prime T cells. Different numbers of MΦs, iDCs or mDCs were incubated for 5 days with 105 allogeneic feline PBMCs at three different stimulator/responder (S:R) ratios, namely, 1:10, 1:33, and 1:100. As can be seen in Fig. 4, mDCs were considerably more powerful at inducing proliferation of allogeneic PBMCs than iDCs (for 1:33 and 1:100 S:R, P < 0.05), although the latter were still better than MΦs (for 1:100 S:R, P < 0.01). At the S:R ratios tested, ΜΦs grown in vitro in the conditions described above were not able to induce MLR.
FIG. 4.
Immunostimulatory function of MΦs, iDCs and mDCs in MLR. Adherent PBMCs were cultured for 7 days with (iDC; ▪) or without (MΦ; ▴) GM-CSF and IL-4. For mDCs (□), LPS was added the last 48 h of culture. Cells were then incubated for 4 days with 105 allogeneic PBMCs at the S:R ratios indicated, and then [methyl-3H]thymidine was added to wells for another 18 h. Results are expressed as the means ± standard errors of triplicates.
Thus, LPS was able to induce feline iDCs to mature into cells that primed T lymphocytes, as also shown for DCs from other animal species (3, 16).
Testing different stimuli to induce DC maturation.
Different maturation stimuli were tested and compared to LPS. The following factors were tried out: (i) poly(I:C), an artificial double stranded RNA which acts by binding to toll-like receptor(s) and that has been shown to induce maturation of human DCs (26); (ii) activated platelets, which have been reported to induce maturation of human DCs by CD40-CD40L interaction (7), and to polarize human plasmacytoid DCs towards the induction of a Th1-type immune response (2); (iii) recombinant human TNF-α, which acts through specific receptors and is very often used in combination with other factors to induce DC maturation in in vivo studies in primates and other species (9, 13); (iv) human IFN-α, either natural or recombinant, which has been shown to induce maturation of human DCs (22).
Preliminary experiments were set up to determine the optimal concentration of each substance in terms of cell viability and mature phenotype. To compare the different maturation stimuli, 20 μg/ml poly(I:C), 5 × 106 feline activated platelets/ml, 100 ng/ml TNF-α, 1,000 U/ml IFN-α, or 50 ng/ml LPS was added to iDC cultures at day 5, and 2 days later, cells were analyzed for the expression of CD14, CD1a, MHC class II, and B7.1; for phagocytosis of FITC-DX during a 45-min incubation; and for the ability to prime allogeneic naïve T cells.
Cells treated with poly(I:C) were visibly different from iDCs, they were more adherent to plastic and highly granular, with large intracellular vacuoles, and were generally bigger than LPS-treated cells (not shown). In five different experiments, poly(I:C) was compared to LPS in inducing DC maturation. Table 1 shows typical results obtained. Poly(I:C) increased MHC class II expression considerably, in some experiments as much as LPS did. B7.1 was also upregulated, showing that costimulatory molecules were expressed on the surface of poly(I:C)-treated cells; in addition, FITC-DX incorporation by these cells was as low as for LPS-treated DCs, in some experiment even lower. Surprisingly, however, when assayed in MLR, poly(I:C)-treated DCs were no more powerful than iDCs in inducing MLR (P > 0.1), while LPS-treated cells were 10 times as effective as iDCs (P < 0.001).
TABLE 1.
Comparative effect of LPS and poly(I:C) on the expression of MHC class II and B7.1 on phagocytic ability and T-cell priming ability of feline DCs
| Stimulus (amt) | MFIa
|
cpmb(mean ± SE) | ||
|---|---|---|---|---|
| MHC class II | B7.1 | FITC-DXc | ||
| None | 20 | 3 | 34 | 1,352 ± 275 |
| LPS (50 ng/ml) | 362 | 25 | 21 | 22,013 ± 1,268 |
| Poly(I:C) (20 μg/ml) | 82 | 14 | 16 | 2,344 ± 654 |
Mean fluorescence intensity (MFI) of each population subtracted from MFI of background (see Materials and Methods). One representative experiment of five is shown.
DCs (104) were incubated for 5 days with 105 allogeneic PBMCs, and then [methyl-3H]thymidine was added to wells for the last 18 h. Results are expressed as the means ± standard errors in cpm of triplicates.
FITC-DX was added to DCs at 200 μg/ml, and DCs were incubated for 45 min at 37°C or on ice (background).
Activated platelets induced iDCs to resemble LPS-treated cells, i.e., they looked smaller, rounder and granular, but platelet-treated DCs showed down-regulated markers and they did not induce MLR (data not shown). DCs treated with human TNF-α or IFNα did not differ from iDCs in any aspect: these factors did not seem to have any effect on feline iDCs, not even at very high concentrations, presumably because they are not cross-species reactive (data not shown).
Thus, in our hands, none of the stimuli tested was as effective as LPS in producing MLR-inducing DCs, although FACS profiles sometimes looked promising.
DISCUSSION
The focus of the present study was to establish culture conditions for feline DCs with the aim of using them in vivo for immunoprophylaxis and/or therapy experiments. To this end, we worked at (i) replacing FCS with another supplement and setting up a standard culture protocol, (ii) characterizing the DCs obtained in the culture conditions established, and (iii) inducing maturation of feline DCs.
Reinjection of DCs cultured in FCS in other animal species resulted in priming of T cells to xenogeneic proteins that clearly limited the use of such DCs and even gave rise to allergic reactions (11, 12, 14). Therefore, we did not use FCS in our cultures. Another reason for not using FCS is that we have noticed in the past that feline PBMCs grown in the presence of FCS and pulsed with [methyl-3H]thymidine exhibited higher proliferation backgrounds than the same grown in the presence of human serum (unpublished observation), probably because most cats used in experimental laboratories are vaccinated by breeders with standard vaccines containing traces of FCS. After trying out feline serum and plasma at different concentrations, to culture feline DCs, we used autologous heparin-containing plasma at a 3% concentration, and feline DCs were obtained from plastic-adherent monocytes in vitro in the presence of GM-CSF and IL-4. Great differences were noticed between DCs differentiated from the same cats in FCS- or autologous plasma-containing media: often, although not always, FCS induced evident cell proliferation but surface markers were generally expressed on the surface at lower levels than in autologous plasma-containing medium.
In our hands, cell yields were limited by the blood volume that could be drawn from cats at any one time (maximum, 35 ml). In addition, the numbers of total PBMCs obtained per ml of blood could vary up to a factor of 10 from one cat to the other and, for the same cat, from one time to the next, although there was a tendency of individual cats to yield certain numbers of PBMCs per ml blood. The ability of adherent cells to survive and differentiate also varied from one experiment to the next, for reasons that we were not able to pinpoint. Incidentally, we found that EDTA-containing autologous plasma was toxic to feline DCs.
To our knowledge, this is the first study attempting to test and compare different agents to induce maturation of feline DCs. LPS turned out to be the most powerful maturation agent, in terms of ability to upregulate MHC class II and B7.1 expression, to downregulate phagocytosis and, above all, to stimulate MLR. From the morphological point of view, LPS induced iDCs to “shrink” and to develop granules, as judged by the light scatter properties of DCs and as seen by light microscopy. LPS-matured DCs looked semiadherent, round and extremely granular (Fig. 1). MHC class II and B7.1 expression was found to be extremely high on DCs matured with LPS, and also CD14 expression was high, while mannose receptor-mediated phagocytosis was downregulated, in agreement with what described for human DCs (20). What is more important in a model with no available maturation markers, LPS-matured DCs were able to induce alloreactivity at S:R ratios ranging from 1:100 to 1:30, depending from the donors (an example is shown in Fig. 4).
All other stimuli tested were disappointing as maturation agents compared to LPS. Recombinant human TNF-α and IFN-α had no effect we could measure on feline DCs. We hypothesized that feline receptors were not able to bind human cytokines, but the reason for the lack of activity was not understood. Activated platelets were toxic at doses of 107/ml or higher. At concentrations around 106/ml, they had visible effects on iDCs such as the induction of shrinking and granules, as judged by light scatter properties (not shown). However, they did not affect the capacity of DCs to activate allogeneic T cells. Poly(I:C) led cells to resemble LPS-matured DCs in terms of surface marker expression and phagocytosis levels (Table 1), but, when tested in MLR, poly(I:C)-treated cells were no better that iDCs.
In the effort of finding features of feline DCs relating to their efficacy as antigen-presenting cells, an attempt was made to correlate the ability of cells to induce MLR in a five-day assay with phenotypic markers that could be determined on the same day as cell harvest, thereby allowing the evaluation of DC quality before injection in vivo. We measured CD14, CD1a, MHC class II, and B7.1 expression and the levels of phagocytosis of FITC-DX and Alexa in parallel with the ability to prime MLR. CD1a expression, which has been previously detected on feline Langerhans cells with the same antibody used here (18), was present on all DCs obtained, both immature and mature, at the same levels, while it was found to be low on MΦs. CD14, which has been reported to decrease on the surface of monocytes during their differentiation into DCs in humans and other species (9, 13), did change between MΦs and iDCs, both in terms of percentage of cells expressing the marker and in intensity of surface expression. Most DCs were positive for CD14, which was detectable at the same levels on MΦs and mDCs. Costimulatory molecule B7.1 was upregulated after LPS treatment and initially looked like an appealing maturation marker, since it has been described to be necessarily expressed on human cells priming an autologous MLR (23). However, it was also upregulated on poly(I:C)-treated DCs although these cells failed to trigger MLR.
Uptake of exogenous antigen is known to be efficiently carried out by iDCs, both by mannose receptor-mediated phagocytosis and by fluid-phase pinocytosis. Consequently, we also compared the ability of mDCs and iDCs to uptake FITC-DX and Alexa. As can be seen in Fig. 3, iDCs rapidly accumulated FITC-DX in their cytoplasm while, as expected, LPS induced DCs to downregulate FITC-DX uptake (Fig. 3).
Taken together, these data show that there does not seem to be any correlation between any of the markers tested and the ability to induce MLR; as an example, only LPS made DCs able to prime allogeneic T cells, but MHC class II and B7.1 were upregulated and FITC-DX uptake was downregulated in both poly(I:C) and LPS-treated DCs. Therefore, to assess the maturation degree of feline DCs, it will be necessary to rely on induction of alloreactivity as a measurement of DC ability to activate naïve T cells.
To sum up, we have set up conditions that allow culture of sufficient numbers of DCs from feline PBMCs that can be used in vivo. DCs cultured following the protocol presented do not make contact with xenoproteins and are, therefore, expected only to present the antigen they will be charged with in possible immunotherapy/vaccination experiments.
Acknowledgments
This work was supported by grants from the Ministero della Salute-Istituto Superiore di Sanità “Programma per l'AIDS,” Rome, Italy.
We thank R&D Systems for their generous gifts of anti-human antibodies and Wayne Tompkins for his generous gift of the anti-B7.1 antibody. We are also grateful to Filippo Belardelli for helpful advice.
REFERENCES
- 1.Bienzle, D., F. Reggeti, M. E. Clark, and C. Chow. 2003. Functional feline dendritic cells derived from blood and bone marrow. Vet. Immunol. Immunopathol. 96:19-30. [DOI] [PubMed] [Google Scholar]
- 2.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305-310. [DOI] [PubMed] [Google Scholar]
- 3.De Smedt, T., B. Pajak, E. Muraille, L. Lespagnard, E. Heinen, P. De Baetselier, J. Urbain, O. Leo, and M. Moser. 1996. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184:1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunham, S. P., and J. Bruce. 2004. Isolation, expression and bioactivity of feline granulocyte-macrophage colony-stimulating factor. Gene 332:97-106. [DOI] [PubMed] [Google Scholar]
- 5.Figdor, C. G., I. J. de Vries, W. J. Lesterhuis, and C. J. Melief. 2004. Dendritic cell immunotherapy: mapping the way. Nat. Med. 10:475-480. [DOI] [PubMed] [Google Scholar]
- 6.Freer, G., W. Florio, B. Dalla Casa, D. Bottai, G. Batoni, G. Maisetta, S. Senesi, and M. Campa. 1998. Identification and molecular cloning of a novel secretion antigen from Mycobacterium tuberculosis and Mycobacterium bovis BCG. Res. Microbiol. 149:265-275. [DOI] [PubMed] [Google Scholar]
- 7.Gatti, E., M. A. Velleca, B. C. Biedermann, W. Ma, J. Unternaehrer, M. W. Ebersold, R. Medzhitov, J. S. Pober, and I. Mellman. 2000. Large-scale culture and selective maturation of human Langerhans cells from granulocyte colony-stimulating factor-mobilized CD34+ progenitors. J. Immunol. 164:3600-3607. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 9.Lu, W., X. Wu, Y. Lu, W. Guo, and J. M. Andrieu. 2003. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 9:27-32. [DOI] [PubMed] [Google Scholar]
- 10.Lu, W., L. C. Arraes, W. T. Ferreira, and J. M. Andrieu. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 10:1359-1365. [DOI] [PubMed] [Google Scholar]
- 11.Mackensen, A., R. Drager, M. Schlesier, R. Mertelsmann, and A. Lindemann. 2000. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol. Immunother. 49:152-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller, G., A. Muller, H. Jonuleit, K. Steinbrink, C. Szalma, L. Paragnik, K. Lingnau, E. Schmidt, J. Knop, and A. H. Enk. 2000. Fetal calf serum-free generation of functionally active murine dendritic cells suitable for in vivo therapeutic approaches. J. Investig. Dermatol. 14:142-149. [DOI] [PubMed] [Google Scholar]
- 13.Nair, S., C. McLaughlin, A. Weizer, Z. Su, D. Boczkowski, J. Dannull, J. Vieweg, and E. Gilboa. 2003. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J. Immunol. 1:6275-6282. [DOI] [PubMed] [Google Scholar]
- 14.Nestle, F. O., S. Alijagic, M. Gilliet, Y. Sun, S. Grabbe, R. Dummer, G. Burg, and D. Schadendorf. 1998. Vaccination of melanoma patients with peptide- or tumor lysate pulsed dendritic cells. Nat. Med. 4:328-332. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill, D., and N. Bhardwaj. 2005. Exploiting dendritic cells for active immunotherapy of cancer and chronic infection. Methods Mol. Med. 109:1-18. [PubMed] [Google Scholar]
- 16.Paillot, R., F. Laval, J.-C. Audonnet, C. Andreoni, and V. Juillard. 2001. Functional and phenotypic characterization of distinct porcine dendritic cells derived from peripheral blood monocytes. Immunology 102:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kampgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods 196:137-151. [DOI] [PubMed] [Google Scholar]
- 18.Saint-André Marchal, I. C., J.-P. Dezutter-Dambuyant, B. J. Martin, et al. 1997. Quantitative assessment of feline epidermal Langerhans cells Br. J. Dermatol. 136:961-963. [PubMed] [Google Scholar]
- 19.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage-colony stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor-a. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto, F., M. Cella, C. Danielli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 12:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto, F., and A. Lanzavecchia. 2002. The instructive role of dendritic cells on T-cell responses. Arthr. Res. 4:S127-S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santini, S. M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 15:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheinecker, C., K. P. Machold, O. Majdic, P. Hocker, W. Knapp, and J. S. Smolen. 1998. Initiation of the autologous mixed lymphocyte reaction requires the expression of costimulatory molecules B7-1 and B7-2 on human peripheral blood dendritic cells. J. Immunol. 161:3966-3973. [PubMed] [Google Scholar]
- 24.Steinman, R. M., and M. Pope. 2002. Exploiting dendritic cells to improve vaccine efficacy. J. Clin. Investig. 109:1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tompkins, M. B., M. F. Bull, J. L. Dow, J. M. Ball, E. W. Collisson, B. J. Winslow, A. P. Phadke, T. W. Vahlenkamp, and W. A. Tompkins. 2002. Feline immunodeficiency virus infection is characterized by B7+CTLA4+ T cell apoptosis. J. Infect. Dis. 185:1077-1093. [DOI] [PubMed] [Google Scholar]
- 26.Verdijk, R. M., T. Mutis, B. Esendam, J. Kamp, C. J. M. Melief, A. Brand, and E. Goulmy. 1999. Polyriboinosinic polyribocytidylic acid (Poly(I:C)) induces stable maturation of functionally active human dendritic cells. J. Immunol. 163:57-61. [PubMed] [Google Scholar]
- 27.Willett, B. J., C. A. Cannon, and M. J. Hosie. 2003. Expression of CXCR4 on feline peripheral blood mononuclear cells: effect of feline immunodeficiency virus infection. J. Virol. 77:709-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, L. J., and T. F. Tedder. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 154:3821-3829. [PubMed] [Google Scholar]
- 29.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]