Abstract
Five Borrelia burgdorferi sensu lato isolates from Missouri are described. This represents the first report and characterization of such isolates from that state. The isolates were obtained from either Ixodes dentatus or Amblyomma americanum ticks that had been feeding on cottontail rabbits (Sylvilagus floridanus) from a farm in Bollinger County, Mo., where a human case of Lyme disease had been reported. All isolates were screened immunologically by indirect immunofluorescence by using monoclonal antibodies to B. burgdorferi-specific outer surface protein A (OspA) (antibodies H3TS and H5332), B. burgdorferi-specific OspB (antibody H6831), Borrelia (genus)-specific antiflagellin (antibody H9724), and Borrelia hermsii-specific antibody (antibody H9826). Analysis of the isolates also involved a comparison of their protein profiles by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Finally, the isolates were analyzed by PCR with six pairs of primers known to amplify selected DNA target sequences specifically found in the reference strain B. burgdorferi B-31. Although some genetic variability was detected among the five isolates as well as between them and the B-31 strain, enough similarities were found to classify them as B. burgdorferi sensu lato.
Considerable controversy exists as to whether Borrelia burgdorferi and Lyme disease (LD) occur in the southern United States, particularly in Missouri (21). There is clinical evidence of LD there (16–19) and strong evidence of B. burgdorferi in nature (9). Some also have proposed that the human cases reported in Missouri are not true LD but are “Lyme disease-like” (8).
Various methods have been used to determine the presence of B. burgdorferi in a geographic area or in a host species. However, the one indisputable method is the isolation of spirochetes from ticks or hosts in nature in Barbour-Stoenner-Kelly (BSK) culture medium and subsequent determination that the spirochete is B. burgdorferi. We sampled ticks and tick hosts in southeastern Missouri from July 1993 through June 1996 in attempts to obtain B. burgdorferi or other spirochetal isolates. A brief preliminary report at the Sixth International Conference on Lyme Borreliosis noted our initial success in isolating in culture the first B. burgdorferi isolates from Missouri (22). Here we present a fuller account and the first descriptions and characterizations of these and additional B. burgdorferi sensu lato isolates from Missouri. The five isolates described in this paper are from one farm in Bollinger County, Mo., where a physician-diagnosed case of LD was reported (17). These five isolates are among 44 that we have currently obtained from eight geographic areas within five counties in southeastern Missouri.
MATERIALS AND METHODS
Spirochetal isolates.
Eastern cottontail rabbits (Sylvilagus floridanus) were collected from September 1993 through September 1995 on a single farm in Bollinger County, which is in southeastern Missouri. Larval and nymphal Ixodes dentatus and larval Amblyomma americanum ticks removed from rabbits were surface sterilized, triturated, and inoculated into BSK II medium (3) containing 0.023% l-cysteine hydrochloride, 0.015% dl-dithiothreitol (Sigma Chemical Co., St. Louis, Mo.), 1 μg of l-glutamine (GIBCO Laboratories, Fairfield, N.J.) per ml, 0.15% soft agarose (Seakem; FMC Bioproducts, Rockland, Maine), 50 μg of rifampin (Sigma) per ml, 20 μg of phosphomycin (Sigma) per ml, and 2.5 μg of amphotericin B (Fungizone; GIBCO) per ml (13, 26). Cultures were incubated in a 5% CO2 atmosphere at 33 to 34°C and were examined for spirochetes by dark-field microscopy twice weekly for 2 weeks and weekly thereafter for 6 weeks.
Monoclonal antibodies.
Spirochetal isolates were screened immunologically by indirect fluorescent-antibody (IFA) analysis with a series of monoclonal antibodies (see Table 1). They included two B. burgdorferi-specific anti-outer surface protein A (OspA) monoclonal antibodies (H3TS and H5332), one B. burgdorferi-specific anti-outer surface protein B (OspB) monoclonal antibody (H6831), a Borrelia (genus)-specific antiflagellin monoclonal antibody (H9724), and a Borrelia hermsii-specific monoclonal antibody (H9826).
TABLE 1.
Immunofluorescent reactivities of spirochetal isolates to monoclonal antibodies
| State, isolate | Tick speciesa | Immunofluorescent reactivity
|
||||
|---|---|---|---|---|---|---|
| OspA
|
OspB (H6831) | Borrelia genus (H9724) | B. hermsii (H9826) | |||
| H3TS | H5332 | |||||
| Missouri | ||||||
| MOD-1 | I. dentatus (2 N) | − | − | − | + | − |
| MOD-2 | I. dentatus (2 N) | − | − | − | + | − |
| MOD-3b | I. dentatus (4 L) | − | − | − | + | − |
| MOD-5 | I. dentatus (N) | − | + | + | + | − |
| MOD-6 | A. americanum (L) | − | − | − | + | − |
| Georgia, BC-1 | I. dentatus (N) | − | − | − | + | − |
N and L, nymphs and larvae, respectively.
In one of three replicates, approximately 15% of spirochetes reacted strongly to monoclonal antibodies H5332, H3TS, and H6831.
SDS-PAGE.
Whole spirochetal lysates were prepared from each BSK II culture for characterization by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Fifty-milliliter aliquots of each spirochetal culture were centrifuged at 10,000 × g for 30 min and the supernatants were removed. The spirochetal pellets were washed three times in 10 ml of phosphate-buffered saline (120 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 10 mM KH2PO4 [pH 7.4]) with 5 mM MgCl2. The pellets were then suspended in 0.2 ml of sterile deionized water and frozen-thawed at −80°C for 30 min five times. After the final thaw, the pellets were vortex mixed and aliquots were taken for protein determination by the method of Bradford (6). SDS-PAGE was carried out by the method of Laemmli (14), with the following modifications. Each spirochetal lysate was mixed with an equal volume of denaturing buffer containing 20% 2-mercaptoethanol. The lysates were then heated at 100°C for 15 min with vigorous vortexing at 5-min intervals. For each sample, a volume containing 30 μg of protein was loaded into a 4% stacking gel and was resolved through a 14% separating gel that had been precooled to 0°C. Low-molecular-weight protein standards (Bio-Rad Laboratories, Richmond, Calif.) were included with each run. Coolant at 0°C was circulated through a Protean II xi cell (Bio-Rad Laboratories) until electrophoresis was complete. The samples were electrophoresed at 80 mA of constant current until the dye front had migrated 10 cm through the separating gel. The gel was stained overnight with 0.2% Coomassie brilliant blue R-250. It was then destained, photographed, and scanned at a wavelength of 600 nm with a Shimadzu densitometer (model CS-9000U) interfaced with a CSTURBO analysis program (Shimadzu Corp., Tokyo, Japan).
PCR.
PCR with six primer pairs was used to amplify known DNA target sequences present in the reference strain B. burgdorferi B-31 (see Table 2). The primers and parameters are listed in a report by Oliver et al. (23). Before PCR amplification, each spirochetal lysate was centrifuged at 600 × g for 15 min to sediment cellular debris. Spirochetal supernatant (0.5 μg of protein) was placed in 10 μl of distilled water and was heated at 100°C for 10 min to inhibit proteolytic activity prior to adding it to the PCR mixture. Ten microliters of this solution was used as template for PCR amplification in a final reaction volume of 50 μl that contained 2.5 U of Taq DNA polymerase, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 200 μM (each) deoxynucleotide triphosphate (dATP, dTTP, dCTP, and dGTP), and 50 pmol of each appropriate primer (23). Amplification was performed with a Perkin-Elmer/Cetus (Norwalk, Conn.) thermal cycler (model 9600). Four pairs of primers (primers 149-319, 149-459, 788-946, and 3′-5′) amplified 170-, 310-, 158-, and 879-bp sequences, respectively, within the B. burgdorferi B-31 strain outer surface protein A (ospA) gene (24). A fifth pair of primers (primers 245 and 855) amplified a 610-bp target sequence found in the flagellin (fla) gene of the B-31 strain (12). A sixth pair of primers (primers 147 and 520) was specific for a 373-bp chromosomal target present in 17 of 18 documented strains of B. burgdorferi worldwide (25). Pure genomic DNA (5 ng) of B. burgdorferi B-31 was used as a positive control for each PCR assay, and sterile distilled water was used as a negative control. PCR-amplified products were electrophoresed in 2% agarose gels, stained with ethidium bromide, and visualized by UV light. Amplification products seen as distinct bands were compared to known standards and documented for permanent record by Polaroid (Cambridge, Mass.) photography of UV-transilluminated gels.
TABLE 2.
Results of PCR assay of spirochetal isolates from Missouri and Georgia with six B. burgdorferi-specific primer pairs
| Strain, date of isolation | Tick | Reactivities of the indicated primer pairs with the following gene sequences:
|
|||||
|---|---|---|---|---|---|---|---|
|
ospA
|
fla (245-855) | Chromosomal (147-520) | |||||
| 788-946 | 149-319 | 149-459 | 3′-5′ | ||||
| MOD-1, September 1993 | I. dentatus, two nymphs | + | + | + | + | + | − |
| MOD-2, September 1993 | I. dentatus, two nymphs | − | + | + | + | + | − |
| MOD-3, September 1993 | I. dentatus, four larvae | − | + | + | + | + | − |
| MOD-5, November 1994 | I. dentatus, one nymph | + | + | + | + | + | + |
| MOD-6, October 1995 | A. americanum, one larva | − | + | + | + | + | − |
| BC-1, February 1993 | I. dentatus, one nymph | − | + | + | + | + | − |
| B-31, reference strain | I. scapularis | + | + | + | + | + | + |
RESULTS
Monoclonal antibodies.
All of the Missouri isolates (Table 1) reacted positively to the Borrelia (genus)-specific antibody (H9724) and failed to react to the B. hermsii-specific antibody (H9826). The MOD-5 isolate reacted to one of the OspA monoclonal antibodies (H5332), but not to the other one (H3TS); however, it reacted to the OspB antibody (H6831). The other isolates (MOD-1, MOD-2, MOD-3, and MOD-6) did not react to any of the antibodies tested except the Borrelia (genus)-specific antibody (H9724). An exception was MOD-3, which in one of the three replicate tests contained approximately 15% of the spirochetes that strongly reacted to the OspA H5332 and H3TS antibodies and the OspB H6831 antibodies. On the basis of the results obtained with the monoclonal antibodies tested, the five isolates can be arranged into two groups: group 1, MOD-1, MOD-2, MOD-3, and MOD-6; group 2, MOD-5 (Table 1). They can be arranged into three groups if the MOD-3 culture contains two strains.
SDS-PAGE.
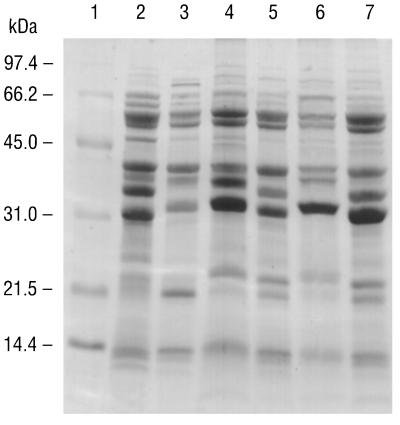
All major proteins of the B. burgdorferi B-31 reference strain, including the 31-kDa OspA, the 34-kDa OspB, and the 41-kDa flagellin proteins, were readily resolved in a Coomassie brilliant blue-stained 14% polyacrylamide gel. The major proteins of the B-31 strain were identical to those reported previously (3, 4). The major proteins of the five Missouri isolates and the B-31 reference strain were compared by SDS-PAGE and by overlaying visible bands on densitometer tracings. The 41-kDa flagellin protein of all five Missouri isolates appeared to be identical to that of the B-31 strain when comparing the migration patterns, which were equivalent. However, the composition of the outer surface proteins of all of the Missouri isolates differed, some only slightly, from that of the B-31 reference strain (Fig. 1). For all of the Missouri isolates except MOD-5 there was a shift in both OspA and OspB proteins to higher molecular masses (≈32 and 35 kDa, respectively, compared to ≈31 and 34 kDa, respectively, for the B-31 strain). For isolate MOD-5, however, only one major Osp band was resolved, and it coincided with the OspA band of the B-31 reference strain; an OspB band was not detectable in the SDS-polyacrylamide gel, but an OspB reaction was obtained by IFA analysis with monoclonal antibody H6831 (Table 1). Lastly, for all Missouri isolates in which it could be resolved, there was a shift in the major low-molecular-mass protein (approximately 21 to 22 kDa) compared to that for the B-31 strain.
FIG. 1.
SDS-PAGE of whole spirochetal lysates. Lane 1, molecular size standards; lane 2, reference strain B. burgdorferi B-31; lane 3, isolate MOD-1; lane 4, isolate MOD-2; lane 5, isolate MOD-3; lane 6, isolate MOD-5; lane 7, isolate MOD-6.
PCR.
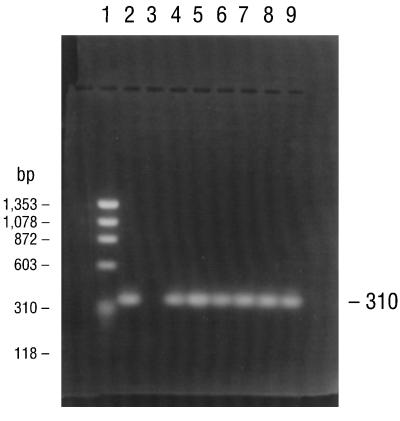
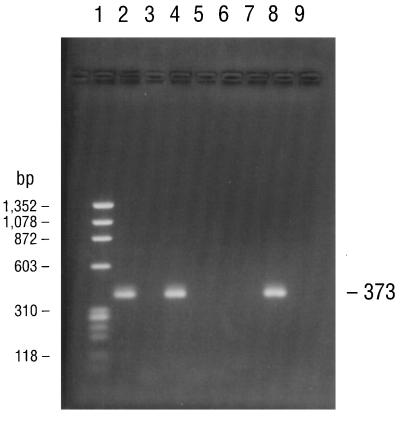
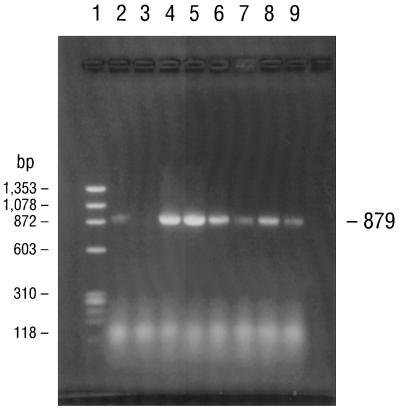
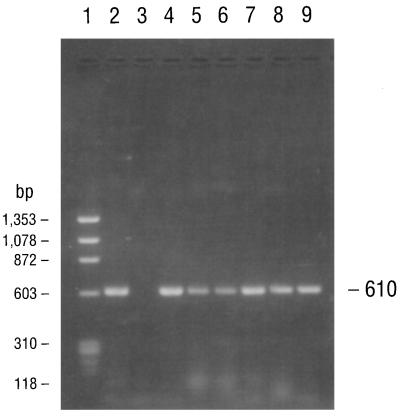
PCR consistently amplified target sequences in DNA from the cultured B. burgdorferi B-31 reference strain when the fla primer pair (primers 245 and 855), the conserved chromosomal primer pair (primers 147 and 520), and all four pairs of ospA primers (primer pairs 788-946, 149-319, 149-459, and 3′-5′) were used (Table 2). PCR also amplified target sequences in DNAs from all of the Missouri isolates when the fla and all ospA primer pairs but not the 788-946 primer pair were used. Isolates MOD-1 and MOD-5 also amplified the latter primer pair. The chromosomal target sequence (primers 147 and 520) was repeatedly amplified and detected in DNA from isolate MOD-5 but not in DNAs from any of the other Missouri isolates. Agarose gel electrophoresis of the PCR-amplified products of the B-31 reference strain and the five Missouri isolates with primers 149-459 and 3′-5′ for the ospA gene, primers 245-855 for the fla gene, and primers 147-520 for the conserved chromosomal gene are illustrated in Fig. 2 to 5, respectively. DNA from the B. burgdorferi B-31 reference strain served as a positive control for PCR and yielded clearly visible amplification products of the expected size, seen as distinct bands after appropriate primers were used and after gel electrophoretic analysis of the products. Negative control reactions did not yield any amplified products.
FIG. 2.
Agarose gel electrophoresis of PCR products amplified with primers for the 310-bp sequence of the B. burgdorferi ospA gene (primers 149 and 459). Lane 1, DNA size standards; lane 2, B. burgdorferi genomic DNA (positive control); lane 3, sterile distilled water (negative control); lane 4, the B. burgdorferi B-31 reference strain; lane 5, isolate MOD-1; lane 6, isolate MOD-2; lane 7, isolate MOD-3; lane 8, isolate MOD-5; lane 9, isolate MOD-6.
FIG. 5.
PCR amplification of a 373-bp conserved chromosomal gene sequence specific for B. burgdorferi by using primers 147 and 520. The electrophoresed products in lanes 1 to 9 are identical to those described in the legend to Fig. 2.
DISCUSSION
The five spirochetal isolates reported here were collected over a 2-year period from ticks feeding on different rabbits at several locations on the same farm. Ticks feeding on other vertebrate species at the farm were analyzed, but only ticks from rabbits were infected with B. burgdorferi. Moreover, two of the five isolates reported here were cultured from larval-stage ticks. Since transovarial transmission of B. burgdorferi is extremely rare (several references cited in reference 21), those larvae almost certainly acquired the infections from the rabbits. We have fed uninfected I. dentatus ticks on naturally infected cottontail rabbits from Missouri, and approximately 64% of them became infected. We have yet to complete the laboratory cycle of transmission of B. burgdorferi back to an uninfected cottontail rabbit, but attempts to do so will be made in the future. Nevertheless, the spirochetes reported here are almost certainly part of a local enzootic cycle involving B. burgdorferi sensu lato, the cottontail rabbit, and I. dentatus. Three of the isolates (MOD-1, MOD-2, and MOD-3) were obtained in September 1993 from ticks collected in August 1993; in May there was a physician-diagnosed case of LD in a member of the family who was living on the farm (17). In August 1995, a second member of the family who lived 6 miles from the farm also was diagnosed as having LD (16). Both of these patients presented classic erythema migrans lesions and other symptoms typical of LD (17). The time and geographic sites of occurrence of the human cases of LD and the presence of an enzootic cycle of the spirochete on the family farm suggest a potential causal association. I. dentatus usually does not bite humans, but recently, there was a report of I. dentatus ticks attached to three humans in three different counties in North Carolina (11). Moreover, a partially fed I. dentatus nymph infected with a novel B. burgdorferi strain was removed from a human in the northeastern United States (2). The spirochetal isolate was similar to previous isolates from I. dentatus ticks feeding on rabbits in that area (1), and these isolates are now recognized as belonging to genomic group 21038, named Borrelia andersonii (15). The isolate from the I. dentatus tick feeding on a human and 6 other novel B. burgdorferi isolates from Ixodes scapularis ticks among the 99 isolates examined in the study reacted with sera from humans with early or late LD. Clearly, novel borreliae occur in ticks feeding on humans, and thus, at least some humans in the northeastern United States are probably being exposed to borreliae other than the classic B-31 type strains that have thus far been isolated from humans. A similar situation may exist in Missouri. Interestingly, the isolate from I. dentatus and the other six novel B. burgdorferi isolates from I. scapularis apparently do not infect C3H or white-footed mice when these rodents are parenterally injected with the isolates (2). The infectivities of the isolates from I. dentatus ticks or rabbits from New York, Georgia, or Missouri for humans are unknown.
Several tick species feed on cottontail rabbits at the farm where we conducted our investigations. Two of them (A. americanum and I. scapularis) commonly bite humans. Indeed, the MOD-6 isolate was derived from an A. americanum larva feeding on a rabbit, and this tick species is an avid biter of humans (10). As noted, I. dentatus ticks sometimes bite humans, but they would not be expected to commonly transmit B. burgdorferi to humans. However, I. scapularis or A. americanum ticks might act as a “bridge” vector of Borrelia species from rabbits to people in a manner similar to that of Ixodes pacificus, which serves as a bridge vector of B. burgdorferi from kangaroo rats (Dipodomys californicus) and dusky-footed wood rats (Neotoma fuscipes) to humans in California (7).
Several attempts to isolate spirochetes from biopsy specimens of erythema migrans lesions on humans from Missouri were unsuccessful when tissues were placed directly in BSK II medium. Perhaps some of the B. burgdorferi strains that infect humans in Missouri are so genetically different that their culture requirements are not met by the BSK medium. There also is the possibility that a unique species that does not grow in BSK medium is involved. A Borrelia species (Borrelia lonestarii) found in A. americanum that does not grow in BSK medium was recently recognized by molecular biology-based techniques (5).
Analyses of the five spirochetal isolates characterized in this paper indicate that they are B. burgdorferi sensu lato. Initial comparisons among isolates from I. dentatus ticks from New York (15), Georgia (20, 23), and Missouri (20) show considerable genetic diversity. Indeed, there appears to be at least two immunological groups (Table 1) and possibly three immunological groups on the basis of the variability detected in the MOD-3 culture. There are at least three genetic groups (Table 2). The isolate from I. dentatus from Georgia (BC-1) is similar immunologically to MOD-1, MOD-2, MOD-3, and MOD-6 but is different from MOD-5. On the basis of PCR analysis, the Georgia BC-1 isolate from I. dentatus is similar to MOD-2, MOD-3, and MOD-6 but different from MOD-1 and MOD-5; the last two are different from each other (Table 2). Further analyses and comparisons of the isolates from I. dentatus from Missouri, Georgia, and New York are under way.
FIG. 3.
PCR amplification of the entire ospA gene (primers 3′ and 5′) of B. burgdorferi. The electrophoresed products in lanes 1 to 9 are identical to those described in the legend to Fig. 2.
FIG. 4.
Agarose gel electrophoresis of PCR products amplified with primers specific for a 610-bp segment of the B. burgdorferi fla gene (primers 245 and 855). The electrophoresed products in lanes 1 to 9 are identical to those described in the legend to Fig. 2.
ACKNOWLEDGMENTS
We thank C. W. Banks for tick and host colony maintenance, Amy Richardson and Peggy Kollars for assistance with the laboratory cultures of spirochetes, and Mary Mesirow for antibody screening. We are grateful to Barbara Johnson, Centers for Disease Control and Prevention, Ft. Collins, Colo.; David Persing, Mayo Clinic, Rochester, Minn.; and Patricia Rosa, Rocky Mountain Laboratories, Hamilton, Mont., for supplying primers for genetic analyses and pure genomic DNA of B. burgdorferi B-31. We are grateful to Thomas G. Schwan, Rocky Mountain Laboratories, Hamilton, Mont., for providing a series of monoclonal antibodies. We thank L. A. Durden for reviewing the manuscript.
The research was supported in part by National Institutes of Health grant R 37A1-24899 to Georgia Southern University and Centers for Disease Control and Prevention cooperative agreement U50/CCU410281 to Georgia Southern University.
REFERENCES
- 1.Anderson J F, Magnarelli L A, LeFebvre R B, Andreadis T G, McAninch J B, Perng G C, Johnson R C. Antigenically variable Borrelia burgdorferi isolated from cottontail rabbits and Ixodes dentatus in rural and urban areas. J Clin Microbiol. 1989;27:13–20. doi: 10.1128/jcm.27.1.13-20.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J F, Flavell R A, Magnarelli L A, Barthold S W, Kantor F S, Wallich R, Persing D H, Mathiesen D, Fikrig E. Novel Borrelia burgdorferi isolates from Ixodes scapularis and Ixodes dentatus ticks feeding on humans. J Clin Microbiol. 1996;34:524–529. doi: 10.1128/jcm.34.3.524-529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G. Immunochemical analysis of Lyme disease spirochetes. Yale J Biol Med. 1984;57:581–586. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Heiland R A, Howe T R. Heterogeneity of major proteins in Lyme disease borrelia: a molecular analysis of North American and European isolates. J Infect Dis. 1985;152:478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- 5.Barbour A G, Maupin G O, Teltow G J, Carter C J, Piesman J. Identification of an uncultivable Borrelia sp. in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 7.Brown R N, Lane R S. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi. Science. 1992;256:1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- 8.Campbell G L, Paul W S, Schriefer M E, Craven R B, Robbins K E, Dennis D T. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990–1993. J Infect Dis. 1995;182:470–480. doi: 10.1093/infdis/172.2.470. [DOI] [PubMed] [Google Scholar]
- 9.Feir D, Santanello C R, Li B W, Xie C S, Masters E, Marconi R, Well G. Evidence supporting the presence of Borrelia burgdorferi in Missouri. Am J Trop Med Hyg. 1994;51:475–482. [PubMed] [Google Scholar]
- 10.Felz M W, Durden L A, Oliver J H., Jr Ticks parasitizing humans in Georgia and South Carolina. J Parasitol. 1996;82:505–508. [PubMed] [Google Scholar]
- 11.Harrison B A, Engber B R, Apperson C S. Ticks (Acari: Ixodida) uncommonly found biting humans in North Carolina. J Vector Ecol. 1997;22:6–12. [PubMed] [Google Scholar]
- 12.Johnson B J B, Happ C M, Mayer L W, Piesman J. Detection of Borrelia burgdorferi in ticks by species-specific amplification of the flagellin gene. Am J Trop Med Hyg. 1992;47:730–741. doi: 10.4269/ajtmh.1992.47.730. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R C, Marek N, Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984;20:1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masters, E. J. 1997. Unpublished data.
- 17.Masters E J, Donnell H D. Lyme and/or Lyme-like disease in Missouri. Missouri Med. 1995;92:346–353. [PubMed] [Google Scholar]
- 18.Masters E J, Donnell H D, Fobbs M. Missouri Lyme disease: 1989 through 1992. J Spirochet Tick-Borne Dis. 1994;1:12–13. [Google Scholar]
- 19.Masters E J, Donnell H D. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990–1993. J Infect Dis. 1996;173:1527–1528. doi: 10.1093/infdis/173.6.1527. . (Letter to the editor.) [DOI] [PubMed] [Google Scholar]
- 20.Mathiesen D A, Oliver J H, Jr, Kolbert C P, Tullson E D, Johnson B J B, Campbell G L, Mitchell P D, Reed K D, Telford III S R, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 21.Oliver J H., Jr Lyme Borreliosis in the southern United States: a review. J Parasitol. 1996;82:926–935. [PubMed] [Google Scholar]
- 22.Oliver J H, Jr, Kollars T M, Jr, Chandler F W, Jr, Masters E J, Lane R S, Persing D H, Duray P. VI International Conference on Lyme Borreliosis. Bologne, Italy: Societa Editrice Esculapio; 1994. Unusual tick isolates of Borrelia burgdorferi from southeast Missouri associated geographically and temporally with human Lyme disease, abstr. 002m. [Google Scholar]
- 23.Oliver J H, Jr, Chandler F W, Jr, James A M, Huey L O, Vogel G N, Sanders F H., Jr Unusual strain of Borrelia burgdorferi isolated from Ixodes dentatus in central Georgia. J Parasitol. 1996;82:936–940. [PubMed] [Google Scholar]
- 24.Persing D H, Telford III S R, Spielman A, Barthold S W. Detection of Borrelia burgdorferi infection in Ixodes dammini ticks with the polymerase chain reaction. J Clin Microbiol. 1990;28:566–572. doi: 10.1128/jcm.28.3.566-572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosa P A, Schwan T G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989;160:1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- 26.Sinsky R J, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]