Abstract
Background
Intracranial Meningeal Sarcomatosis (MS) is a rare neoplasm of the central nervous system (CNS), characterized by diagnostic challenges and a poor prognosis. This study aimed to utilize Surveillance, Epidemiology, and End Results (SEER) population data to identify prognostic factors and develop a nomogram for overall survival (OS) in patients with intracranial malignant MS between 2000 and 2021.
Methods
Data of 43 patients diagnosed with malignant MS extracted from the SEER registry from 2000 to 2021 were analyzed. Univariate and multivariable Cox regression analyses were performed to examine factors influencing OS. Nomogram for predicting 1-, 3-, 5- years overall survival probability was created based on the multivariate Cox regression model. Additionally, a literature review of intracranial MS was carried out.
Results
A total of 43 patients with malignant MS, spanning all ages, were identified from 2000 to 2021. The gender distribution was 62.8% female (27 patients) and 37.2% male (16 patients). White patients constituted 67.4% of the cohort, and Spanish-Hispanic-Latino patients made up 93% of the total population. Five variables (age, sex, grade, total number of in situ/malignant tumors, surgical resection combined radiation) were brought into multivariate Cox regression to identify the independent prognostic factors for OS. A comprehensive model was developed utilizing four predictors identified through multivariate Cox regression analysis. Additionally, there were 13 literatures accessible with intracranial MS in previous studies for review.
Conclusion
This SEER study identifies important prognostic factors for malignant MS. While suggesting benefits of comprehensive treatment, the findings are limited by sample size and require validation in larger, prospective multicenter studies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-03475-2.
Keywords: Intracranial meningeal sarcomatosis, SEER, Prognosis, Overall survival, Treatments
Introduction
Primary intracranial sarcomatosis are rare central nervous system (CNS) tumors [1, 2]accounting for approximately 0.1–6% of all intracranial tumors [3, 4]. These tumors originate from mesenchymal progenitor cells within the meningeal covering of the brain and along perivascular Virchow-Robin spaces [5]. Intracranial meningeal sarcomas represent a particularly aggressive subset of neurological malignancies, with limited evidence-based treatment options. The clinical course is typically short, with rapid progression often due to the intractable nature of the tumor or its swift recurrence following neurosurgical resection. Meningeal sarcomatosis (MS) were defined as distinct from tumors arising in other parts of the body and brain that become disseminated through the meninges and produce meningeal carcinomatosis or gliomatosis [6]. MS, also termed as meningeal-based sarcoma, is a highly aggressive malignancy originating in the meninges, the protective membranes surrounding the brain and spinal cord. In contrast to the typically benign meningiomas, MS exhibit malignant behavior, marked by rapid proliferation, local invasiveness, and frequent recurrence. These tumors demonstrate a wide range of histological features, often resembling other sarcomatosis, including fibrosarcomas or malignant fibrous histiocytomas. Clinical presentation varies depending on tumor size, anatomical location, and biological aggressiveness, with prevalent symptoms including headache, nausea, seizures, and focal neurological deficits. Given their rarity, diagnosis can be challenging, often requiring a combination of advanced imaging modalities, histopathological examination, and molecular analysis. Treatment usually involves a multimodal approach, incorporating surgical resection, radiotherapy, and chemotherapy. However, the prognosis remains poor due to the tumor’s aggressive nature and high recurrence rates. Ongoing research is focused on developing novel therapeutic strategies aimed at improving survival outcomes and quality of life for affected patients.
The objective of this study is to utilize United States Surveillance, Epidemiology, and End Results (SEER) population data to analyze patients with malignant MS between 2000 and 2021.
Methods
Study population
The study utilized accessible clinical data from SEER*Stat software. Given that the SEER database offers publicly accessible research data, our institutional review board has exempted the SEER database from review and has waived the requirement for informed consent. The data with malignant MS with type “9539/3” in the ICD-O-3 Hist/behav, malignant were screened. Finally, 43 patients were enrolled in the study.
Data collection
Variables were extracted, such as patient age, gender, race, origin recode, marital status at diagnosis, tumor size, tumor grade, primary site, laterality, summary stage 2000 (1998–2017), total number of in situ/malignant tumors, first malignant primary indicator, surgery status, radiation, chemotherapy, overall survival (OS) time and status. Patients’ races included white, black, and the others (American Indian/Alaska Native, Asian or Pacific Islander) groups. DSW group were defined as patients whose marital status divorced, separated, and widowed according to previous literature [7, 8]. We categorized the largest linear tumor size as ≤ or > 40 mm and age as < or ≥ 60 years old on the basis of the optimal cutoff value determined using the survminer R package. Tumor size was categorized as: 0–40 mm, 40 + mm, unknown. Primary Site of tumors included C70.0-Cerebral meninges; C70.9-Meninges, NOS; C71.1-Frontal lobe. Tumor grade included Well differentiated; Grade I Moderately differentiated; Grade II; Undifferentiated; anaplastic; Grade IV; Unknown. Total number of in situ/malignant tumors was categorized as 1 and > 1 groups.
Statistical analysis
The software package R 4.2.0. were used for data analysis. Frequencies and percentages were used to summarize categorical variables. For continuous variables, the mean and standard deviation were reported if the data were normally distributed, while median and interquartile range were used for non-normally distributed data. Nominal data comparisons employed the chi-square test or Fisher’s exact test. Differences between two groups were analyzed using the t-test, Wilcoxon rank sum test or chi-square test. A p-value of less than 0.05 was considered statistical significance.
To maintain statistical power given the small sample size, cases with partial missing data were not excluded from the analysis. For non-outcome variables with missing values, missing entries were categorized as “unknown” rather than being excluded through listwise deletion or imputed. For survival analysis, vital status and survival time were complete for nearly all cases. Standard right-censoring was applied for patients who were alive at the last follow-up date, in accordance with conventional survival analysis methodology.
Univariate Cox regression analysis was conducted to identify potential prognostic variables. Variables (P-value < 0.05) in univariate Cox regression were brought into multivariate Cox regression to identify the independent prognostic factors. Nomogram for predicting 1-, 3-, 5- years overall survival probability was created based on the multivariate Cox regression model. Additionally, the receiver operating characteristic (ROC) curve and the area under the curve (AUC) was used to evaluate the model’s discrimination ability. The uniformity between the nomogram and observed outcomes was assessed via Calibration curves. To internally validate the model and quantify its predictive performance, bootstrap resampling with 1000 iterations was performed to estimate optimism-corrected metrics.
Previous literature summary
A search of electronic databases, PubMed, was conducted from their inception to September 2024 to explore the previous study on this disease. Primary search terms included “meningeal sarcomatosis”. The flowgram of process is shown in Figure S1 and 13 literature were finally included in the further review [4, 6–19].
Results
Baseline characteristics of the study population
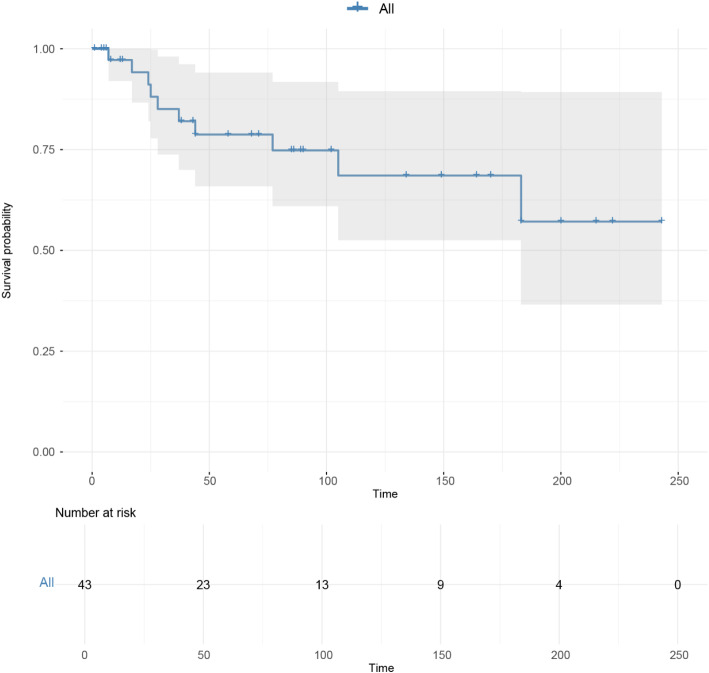
A total of 43 patients with malignant MS, spanning all ages, were identified from 2000 to 2021. Median age of patients at diagnosis was 68 years. Of these patients, 37.2% were under 60 years old, and 62.8% were 60 years or older. The gender distribution was 62.8% female (27 patients) and 37.2% male (16 patients). White patients constituted 67.4% of the cohort, and Spanish-Hispanic-Latino patients made up 93% of the total population. In terms of marital status, 20.9% were married, and 53.5% were single. Tumor size was ≤ 40 mm in 16.3% of patients and > 40 mm in 32.6% of patients. Table 1 presents the characteristics of the study population. Figure 1 presents the overall survival situation of patients with malignant MS.
Table 1.
Distribution of demographics, tumor, and treatment characteristics of meningeal sarcomatosis
| Parameters | Total (N = 43) | Total (N = 43) | |
|---|---|---|---|
| Age, median (range) | 68 (52.50–73.50) | Grade (%) | |
| Age, categorized | Well differentiated; Grade I | 1 (2.3%) | |
| < 60 years, n (%) | 16 (37.2%) | Moderately differentiated; Grade II | 5 (11.6%) |
| ≥ 60 years, n (%) | 27 (62.8%) | Undifferentiated; anaplastic; Grade IV | 3 (7.0%) |
| Gender | Unknown | 34 (79.1%) | |
| Male, n (%) | 16 (37.2%) | Primary site (%) | |
| Female, n (%) | 27 (62.8%) | C70.0—Cerebral meninges | 38 (88.4%) |
| Race | C70.9—Meninges, NOS | 4 (9.3%) | |
| White, n (%) | 29 (67.4%) | C71.1—Frontal lobe | 1 (2.3%) |
| Black, n (%) | 11 (25.6%) | Laterality (%) | |
| Others, n (%) | 3 (7.0%) | Left—origin of primary | 11 (25.6%) |
| Spanish-Hispanic-Latino | Right—origin of primary | 21 (48.8%) | |
| No, n (%) | 3 (7.0%) | Not a paired site | 8 (18.6%) |
| Yes, n (%) | 40 (93.0%) | Paired site | 3 (7.0%) |
| Marital status at diagnosis | Summary stage 2000 (1998–2017) | ||
| Married, n (%) | 9 (20.9%) | Distant | 3 (7.0%) |
| Single, n (%) | 23 (53.5%) | Localized | 20 (46.5%) |
| Divorced/widowed/separated, n (%) | 6 (14.0%) | Regional | 11 (25.6%) |
| Unknown | 5 (11.6%) | Unknown/unstaged | 9 (20.9%) |
| Tumor size | Total number of in situ/malignant tumors | ||
| ≤ 40 mm, n (%) | 7 (16.3%) | 1 | 27 (62.8%) |
| > 40 mm, n (%) | 14 (32.6%) | > 1 | 16 (37.2%) |
| Unknown, n (%) | 22 (51.1%) | Status | |
| First malignant primary indicator | Dead | 26 (60.5%) | |
| No, n (%) | 12 (27.9%) | Live | 17 (39.5%) |
| Yes, n (%) | 31 (72.1%) | Follow-up months, median (IQR) | 68.00 (15.00, 119.50) |
Fig. 1.
Overall survival situation of patients with malignant meningeal sarcomatosis
Management patterns of patients with MS
In Table 2, gross total resection (GTR) was performed in 45.6% (20 patients), radiation therapy in 41.9% (18 patients), and chemotherapy in 0%. Partial resection plus radiation and GTR plus radiation is 11.6% and 23.2%.
Table 2.
Management patterns of patients with meningeal sarcomatosis
| Characteristics | Total (%) |
|---|---|
| Radiotherapy (RT) | |
| No/unknown | 25 (58.1%) |
| Yes | 18 (41.9%) |
| Chemotherapy (CT) | |
| No/unknown | 43 (100%) |
| Surgery | |
| No surgery and biopsy only | 9 (20.9%) |
| Yes | |
| Partial resection | 14 (32.6%) |
| Gross total resection | 20 (45.6%) |
| Treatment comparison | |
| None | 6 (14.0%) |
| Only RT | 3 (7.0%) |
| Only partial resection | 9 (21.0%) |
| Only gross total resection | 10 (23.2%) |
| Partial resection + RT | 5 (11.6%) |
| Gross total resection + RT | 10 (23.2%) |
Feature selection
Univariate Cox regression were used to determine variables (P-value < 0.05) for overall survival in further analysis. Then, through collinearity analysis, it was found that the total number of in situ/malignant tumors and first malignant primary indicator exhibited collinearity. Therefore, first malignant primary indicator will not be included in subsequent multivariate Cox analysis. Finally, five variables (age, sex, grade, total number of in situ/malignant tumors, surgical resection combined radiation) were brought into multivariate Cox regression to identify the independent prognostic factors for overall survival. (Table 3; Fig. 2).
Table 3.
Univariate Cox regression analysis of factors associated with overall survival of meningeal sarcomatosis
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Age, yrs | 1.05 (1.02, 1.09) | 0.003 |
| Gender | ||
| Female | – | – |
| Male | 2.46 (1.07, 5.66) | 0.034 |
| Race | ||
| Asian or Pacific Islander | – | – |
| Black | 1.02 (0.26, 3.96) | 0.979 |
| White | 0.76 (0.22, 2.61) | 0.657 |
| Spanish-Hispanic-Latino | ||
| No, n (%) | – | – |
| Yes, n (%) | 0.67 (0.09, 5.06) | 0.701 |
| Marital status at diagnosis | ||
| Married | – | – |
| Divorced/single/widowed | 0.72 (0.30, 1.73) | 0.464 |
| Unknown | 1.09 (0.36, 3.33) | 0.879 |
| Primary site | ||
| Cerebral meninges | – | – |
| Meninges, NOS | 0.80 (0.19, 3.45) | 0.770 |
| Frontal lobe | 0.00 (0.00, Inf) | 0.998 |
| Grade | ||
| Well differentiated; Grade I | – | – |
| Moderately differentiated; Grade II | 8.25 (1.50, 45.3) | 0.015 |
| Undifferentiated; anaplastic; Grade IV | 1.37 (0.40, 4.63) | 0.617 |
| Unknown | 1.03 (0.11, 10.0) | 0.981 |
| Laterality | ||
| Only one | – | – |
| Two | 3.45 (0.97, 12.3) | 0.055 |
| Stage | ||
| Localized/regional | – | – |
| Distant | 0.53 (0.12, 2.39) | 0.410 |
| Unknown/unstaged | 0.25 (0.04, 1.62) | 0.147 |
| Tumor size | ||
| ≤ 40 mm | – | – |
| > 40 mm | 0.54 (0.20, 1.45) | 0.222 |
| Unknown | 0.46 (0.17, 1.22) | 0.119 |
| Surgical resection combined radiation | ||
| No | – | – |
| Surgical resection only | 0.80 (0.21, 3.00) | 0.736 |
| Radiation only | 6.24 (1.16, 33.5) | 0.033 |
| Both | 1.60 (0.45, 5.70) | 0.466 |
| First malignant primary indicator | ||
| No | – | – |
| Yes | 0.19 (0.08, 0.47) | < 0.001 |
| Total number of in situ/malignant tumors | ||
| 1 | – | – |
| > 1 | 3.24 (1.43, 7.35) | 0.005 |
Bold values indicate p-values < 0.05 in the univariate analysis
Fig. 2.
Feature selection process of nomogram. A Collinearity analysis of variables; B The relationship of the total number of in situ/malignant tumors and first malignant primary indicator; C Multivariate Cox analysis to identify the independent prognostic factors
Nomogram building and validation
A comprehensive model was developed utilizing four predictors identified through multivariate Cox regression analysis. To enhance the accessibility of the model, it was transformed into the form of a nomogram (Fig. 3A), which are recognized as crucial tools in modern medical decision-making, with a graphical representation of statistical prediction models. Subsequently, ROC curve was conducted to assess the model’s discrimination performance (Fig. 3B). In addition, the calibration curve for overall survival probability also indicated a good uniformity between the nomogram and observed outcomes (Fig. 3C). Internal validation was performed using 1000 bootstrap resamples and the results reflects good discriminative ability, robustness and reliability of the model (Table S1).
Fig. 3.
Nomogram Building and Validation. A Nomogram for 1-, 3-, 5-overall survival in patients with malignant meningeal sarcomatosis; B ROC curve conducted to assess the model’s discrimination performance; C Calibration curves for overall survival probability
Previous study accessible on MS
Table 4 show the previous study on MS and the information of patients in these previous study and data is also extracted for review.
Table 4.
Previous study accessible on meningeal sarcomatosis
| Author | PMID | Year | Case number | Description (keywords) | Status | Time (months) | Treatment | Age | Sex | Grade | Total number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kayihan Uluc et al. | 15015662 | 2004 | 1 | a rare case of primary leptomeningeal sarcomatosis in a 20-year-old male. The patient presented with intractable seizures and progressive dementia, and MRI findings showed diffuse leptomeningeal thickening, enhancement especially in the basal cisterns and multiple cystic formations in the brain stem, temporal lobes and basal ganglia. | Alive | 3 | Radiation only | 20 | Male | Unknown | > 1 |
| Arkadiusz Weglewski et al. | 12910846 | 2003 | 1 | a 40-year-old woman with primary leptomeningeal sarcomatosis, initially presenting with pseudotumor cerebri-like symptoms, undiagnosed by neuroimaging, and ultimately leading to her death within eight months. | Death | 8 | No | 40 | Female | Unknown | > 1 |
| Büttner et al. | 11508818 | 2001 | 1 | An 8-year-old girl with persistent headaches and vomiting was diagnosed with primary leptomeningeal sarcomatosis after open biopsy. | Death | 0.5 | Surgical resection only | 8 | Female | Unknown | 1 |
| Alive | 40 | Both | 6 | Female | Unknown | 1 | |||||
| Pfluger et al. | 9106300 | 1997 | 1 | a case of primary leptomeningeal sarcomatosis, several MRI examinations over the course of almost a year were unhelpful, despite severe neurological complaints. | Alive | 5 (–) | Both | 6 | Female | Unknown | 1 |
| Death | 0.5 | Surgical resection only | 8 | Female | Unknown | 1 | |||||
| Wakabayashi et al. | 15726793 | 1997 | 1 | a 10-year-old girl with a primary leptomeningeal tumor developed primary meningeal meningiomatosis, confirmed at autopsy through characteristic cellular markers and morphology. She presented with a 5-week history of increased intracranial pressure, progressive cranial nerve deficits, and spinal compression signs. | Death | 0.5 | Radiation only | 10 | Female | Unknown | 1 |
| Słowiński et al. | 8705280 | 1995 | 1 | A case of 56-year-old male with sarcomatosis of leptomeninges as well as of the brain and spinal cord coexisting with Recklinghausen’s neurofibromatosis is presented. | Death | 7 | No | 56 | Female | Unknown | 1 |
| Vieweg et al. | 8309498 | 1993 | 1 | Primary malignant mesenchymal tumours were seen in 2.4 per cent of all autopsy cases with primary CNS-tumours. The rare disease on the basis of the case history of a 26-year-old patient were discussed. | Death | 8 | Surgical resection only | 26 | Male | Unknown | 1 |
| Thibodeau et al. | 3357038 | 1988 | 1 | a case of primary leptomeningeal sarcomatosis in a 50-year-old man who presented with progressive deficits involving multiple cranial nerves and spinal roots. | Alive | – | Both | 50 | Male | Unknown | > 1 |
| Lukes et al. | 6830462 | 1983 | Meningeal sarcomatosis: rare, diffuse meningeal invasion by sarcoma, mimicking chronic meningitis. Patient: 28-year-old woman, severe headache, diplopia, photophobia. Pathology: Two distinct astrocytomas. Outcome: Rapid decline, death. Diagnosis: Difficult without histology. | Death | 2 | No | 28 | Female | Unknown | 1 | |
| Budka et al. | 56434 | 1975 | 6 | 6 autopsy cases of primary leptomeningeal sarcomatosis are presented as a distinct nosological entity with a variable clinical picture and morphology in 5 males and 1 female. | Death | 6 | No | 47 | Female | Unknown | > 1 |
| Death | 2 | No | 60 | Male | Unknown | 1 | |||||
| Death | 4 | Radiation only | 1 | Male | Unknown | > 1 | |||||
| Death | 0.5 | Surgical resection only | 2 | Male | Unknown | 1 | |||||
| Death | 2 | Surgical resection only | 18 | Male | Unknown | 1 | |||||
| Death | 2 | No | 21 | Male | Unknown | 1 | |||||
| Martin | 5437591 | 1970 | 1 | A 13-year-old male was hospitalized due to headaches and stomach pain. He died after respiratory arrest two months and ten days after admission. A biopsy revealed leptomeningeal sarcoma lesions. | Death | 2 | No | 13 | Male | Unknown | 1 |
| Onofrio et al. | 13940171 | 1962 | 12 | Primary leptomeningeal sarcomas in 12 Mayo Clinic patients had varied symptoms due to multicentric origin, with diffuse or sporadic tumors sometimes leading to dual diagnoses. Patients aged 21 months to 51 years often faced diagnostic confusion with medulloblastoma or other tumors. Reticuloendothelial cell sarcomas had shorter courses than fibrosarcomas, with symptoms lasting from 3 weeks to 6 years. Histologically, tumors showed fibroblastic and reticuloendothelial elements, originating from arachnoid and pia cells or blood vessel walls. | Death | 36 | Both | 4 | Female | Unknown | 1 |
| Death | – | No | 23 | Male | Unknown | > 1 | |||||
| Death | 4 | Surgical resection only | 51 | Male | Unknown | > 1 | |||||
| Death | 1 | Surgical resection only | 18 | Male | Unknown | > 1 | |||||
| Death | 0.25 | Surgical resection only | 22 | Male | Unknown | > 1 | |||||
| Death | – | No | 6 | – | Unknown | > 1 | |||||
| Death | 0.5 | Surgical resection only | 2 | Male | Unknown | > 1 | |||||
| Death | 13 | Both | 25 | Male | Unknown | – | |||||
| Death | 4 | Surgical resection only | 4 | Male | Unknown | – | |||||
| Death | 3 | Both | 2.5 | – | Unknown | > 1 | |||||
| Death | 0.5 | Surgical resection only | 14 | Female | Unknown | > 1 | |||||
| Death | – | No | 15 | Male | Unknown | > 1 | |||||
| Griepentrog et al. | 12986834 | 1952 | 1 | The patient, aged 45, suffered from diffuse meningeal sarcomatosis, presenting symptoms such as mood changes, dizziness, and visual disturbances. The condition progressed, leading to severe neurological impairments and ultimately resulting in death due to respiratory paralysis caused by the tumor. | Death | 8 | No | 45 | Female | Unknown | 1 |
Discussion
Primary intracranial MS is a rare and highly aggressive neurological malignancy characterized by limited evidence-based therapeutic guidelines. Research in this area has been particularly challenging due to the rarity of the condition, the lack of established treatment protocols, and the scarcity of reported cases. Clinical presentations are highly variable, and diagnosis is primarily made through histopathological examination, typically via biopsy or autopsy. The clinical progression is often rapid, and there is insufficient data on the efficacy of standard therapies, such as radiotherapy. This study represents the most comprehensive analysis of these patients in the existing literature. Our findings revealed that prognosis is significantly associated with age, gender, tumor grade, therapeutic regimen, and tumor multiplicity.
The diagnosis of intracranial MS is challenging. Although CT and MRI imaging do not offer a definitive diagnosis for MS, they can provide valuable information regarding the tumor’s extent and meningeal involvement. The lack of pathognomonic imaging features distinguishing MS from other solid intracranial masses or meningeal processes necessitates histopathological confirmation through brain biopsy [4]. A previous study described a primary leptomeningeal sarcoma that exhibited hyperperfusion and elevated choline and lactate levels [20]. These findings may offer some diagnostic insight, although further research is needed to confirm their diagnostic utility.
Current evidence suggests that this tumor presents predominately as a pediatric disease [3]. Although it can occur in any age group. We observed a pattern where older patients exhibited shorter survival durations compared to younger patients. Additionally, male patients demonstrated a less favorable prognosis than female patients. Central nervous system tumours are more common in male [21, 22]. Histological grading significantly influenced disease progression, with grade II tumors exhibiting more aggressive clinical behavior than grade I lesions. High-grade tumors (grades III-IV) were characterized by rapid proliferative activity, frequent recurrence, and elevated metastatic potential [23]. Drawing from past experience, tumors of a higher grade tend to carry a poor prognosis than those of a lower grade [24]. Contrary to expectations, our statistical data suggested that the risk of worse prognosis were not apparent in grade IV than grade 1&2. A potential reason for this unexpected result could be the limited number of grade IV cases available for analysis, which might have skewed the results. The complex prognosis of high-grade tumors is affected by many factors such as tumor size, differentiation degree, stage and treatment response. This needs to investigate for further. Notably, a robust correlation was observed between the number of intracranial tumors and survival rates, with patients presenting with multiple simultaneous MS experiencing significantly lower survival rates compared to those with a single.
From a therapeutic perspective, surgical treatment alone has been associated with more favorable outcomes, although these findings did not reach statistical significance. Nevertheless, extensive experience in tumor management suggests that surgical resection is crucial for maximizing patient survival, whereas radiation therapy alone has been linked to shorter survival durations. Given that many patients are diagnosed at a young age, concerns arise regarding the potential for late toxic effects from radiation, particularly at the doses typically used in treatment [3]. These findings underscore the significant long-term risks associated with radiation therapy, particularly in pediatric populations. Therefore, a multimodal treatment approach, with surgery as a central component, remains essential. In this study, the combination of surgical resection and radiotherapy did not result in a statistically significant improvement in overall prognosis.
The initial treatment of intracranial MS typically involves surgical resection to achieve immediate decompression of critical structures and to establish a definitive pathological diagnosis. Clinical evidence indicates substantially elevated recurrence rates following resection alone. Adjuvant radiosurgery demonstrates potential for improving disease control. Additionally, stereotactic radiosurgery can be considered for lesions that are not amenable to surgical resection. Chemotherapy remains a viable treatment option for both primary and recurrent MS, particularly in cases where surgical or radiosurgical interventions are insufficient or contraindicated. Cytoxic drugs are already being used in front-line therapy [25].
Literature review
Of the 13 reported cases, the age ranged from 8 to 56 years old. Patients suffer from complex symptoms, including seizures, dementia, pseudotumor cerebri-like symptoms, persistent headaches and vomiting, photophobia, progressive cranial nerve deficits, and spinal compression signs, severe neurological impairments. Patients always present increased intracranial pressure. Then, the condition progressed, leading to severe neurological impairments and ultimately resulting in death. MRI findings show diffuse meningeal thickening. However, there are no specific imaging criteria to distinguish MS from other solid brain tumors or other neoplastic or inflammatory MS, and brain biopsy is essential.
Limitations
This study is subject to several limitations. First, as a retrospective analysis, it is inherently constrained by the nature of the data, such as geographic or reporting biases inherent to the regions covered by SEER, which can introduce biases and limit the generalizability of the findings. Second, the SEER database lacks comprehensive information on patients’ physical status and associated complications, which may affect treatment decisions, particularly in elderly patients who are more likely to opt for non-invasive therapies. Third, due to the extremely low incidence of malignant MS, our study included only 43 cases, which limits the statistical power of multivariate analyses and increases the risk of overfitting. Consequently, the generalizability of our proposed nomogram is also limited. Developed from this small single-cohort without external validation, the nomogram should be considered exploratory and hypothesis-generating. Future studies with larger, multicenter datasets are needed to validate and improve the robustness and clinical applicability of the predictive model. Fourth, A further limitation is that the SEER database lacks centralized pathological or imaging confirmation, relying instead on diagnoses reported by individual hospitals. Although we used specific ICD-O-3 codes to reduce misclassification, variability in diagnostic criteria across institutions may still affect case inclusion and the reliability of prognostic interpretations. Fifth, although the SEER database provides a valuable resource for studying rare diseases in a population-based context, its limitations such as the absence of critical clinical parameters, including patient performance status, surgical details, radiation dosage, and chemotherapy regimens should be considered when interpreting the results. Finally, due to the rarity of malignant MS and our limited sample size, the multivariable Cox analysis may be underpowered and prone to wide confidence intervals or overfitting. We used a standard Cox model to maintain simplicity and reduce instability risk in small datasets. As such, our findings should be considered exploratory, and further validation in larger, multicenter cohorts is necessary. Despite these constraints, our SEER analysis offers important insights into the prognostic evaluation of MS and lays the groundwork for future prospective clinical studies comparing different treatment approaches.
Conclusion
This study represents the first population-based analysis of malignant MS using the SEER database. We identified four factors associated with poorer prognosis: older age, male sex, high-grade tumors, and multiple primary tumors. Radiation therapy alone was linked to shorter survival duration, although this finding may be influenced by treatment selection bias. While our findings provide preliminary insights into the prognostic landscape of this rare malignancy, their clinical applicability is limited by the small sample size and retrospective nature of the data. Early diagnosis and accurate prognostic assessment remain important, and our results highlight the potential value of comprehensive treatment approaches. Further prospective, multicenter studies are warranted to validate these findings and guide clinical decision-making.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- CNS
Central nervous system
- SEER
Surveillance, epidemiology and end results
- OS
Overall survival
- GTR
Gross total resection
Author contributions
Xiaoyu Ji and Siyuan Yang wrote the main manuscript. Mingzhe Sun and Tong Wang contributed the data collection. Xuebo Sun, Dejing Cheng and Chengyuan Ji reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
The dataset from the SEER database that was generated and/or analyzed during the current study is available in the SEER dataset repository (https://seer.cancer.gov/). The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study is a retrospective analysis involving data from public databases. Therefore, Ethical approval wasn’t applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Siyuan Yang, Xiaoyu Ji and Tong Wang contributed equally to this work.
Contributor Information
Xuebo Sun, Email: sunxuebosz@163.com.
Dejing Cheng, Email: dejingcheng163@163.com.
Chengyuan Ji, Email: jcy87@126.com.
References
- 1.Dn L, et al. The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro-oncology. 2021;23:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Y-X, et al. Primary intracranial sarcomas: a clinicopathological investigation. Front Oncol. 2023;13: 1195467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.M C, Ga K. Recurrent meningeal sarcoma successfully treated with stereotactic radiosurgery. J Neurosurg Pediatr. 2012;10:434. [DOI] [PubMed] [Google Scholar]
- 4.Büttner A, Pfluger T, Weis S. Primary meningeal sarcomas in two children. J Neurooncol. 2001;52:181–8. [DOI] [PubMed] [Google Scholar]
- 5.Jc L, et al. Primary intracranial sarcomas with DICER1 mutation often contain prominent eosinophilic cytoplasmic globules and can occur in the setting of neurofibromatosis type 1. Acta Neuropathol. 2019;137:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onofrio BM, Kernohan JW, Uihlein A. Primary meningeal sarcomatosis. A review of the literature and report of 12 cases. Cancer. 1962;15:1197–208. [DOI] [PubMed] [Google Scholar]
- 7.Deng Z, Li X, Yang J, Yu H, Zhang N. Marital status independently predicts glioma patient mortality: a surveillance, epidemiology, and end results (SEER) analysis. World Neurosurg. 2021;152:e302-12. [DOI] [PubMed] [Google Scholar]
- 8.Ji X, et al. Prognostic factors and nomogram for malignant brainstem ependymoma: a population-based retrospective surveillance, epidemiology, and end results database analysis. Cancer Med. 2025;14: e70564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Słowiński J, Adamek D, Krygowska-Wajs A, Rudzińska M, Kałuza J. Sarcomatosis of leptomeninges, brain and spinal cord coexisting with von recklinghausen’s neurofibromatosis. A case report and review of the literature. Folia Neuropathol. 1995;33:135–40. [PubMed] [Google Scholar]
- 10.Uluc K, et al. Primary leptomeningeal sarcomatosis; a pathology proven case with challenging MRI and clinical findings. J Neurooncol. 2004;66:307–12. [DOI] [PubMed] [Google Scholar]
- 11.Weglewski A, Juryńczyk J, Papierz W. [Primary leptomeningeal sarcomatosis. Case report]. Neurol Neurochir Pol. 2003;37:251–8. [PubMed] [Google Scholar]
- 12.Pfluger T, et al. MRI of primary meningeal sarcomas in two children: differential diagnostic considerations. Neuroradiology. 1997;39:225–8. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi K, et al. Primary leptomeningeal meningiomatosis with widespread whorl formation. Brain Tumor Pathol. 1997;14:139–43. [DOI] [PubMed] [Google Scholar]
- 14.Vieweg U, Synowitz HJ, Minda R, Dietzmann K, Schmitt JA. [Primary leptomeningeal sarcomatosis in a 26-year-old patient]. Neurochirurgia (Stuttg). 1993;36:213–5. [DOI] [PubMed] [Google Scholar]
- 15.Thibodeau LL, Ariza A, Piepmeier JM. Primary leptomeningeal sarcomatosis. Case report. J Neurosurg. 1988;68:802–5. [DOI] [PubMed] [Google Scholar]
- 16.Lukes SA, Wollmann R, Stefannson K. Meningeal sarcomatosis and multiple astrocytomas. Arch Neurol. 1983;40:179–82. [DOI] [PubMed] [Google Scholar]
- 17.Budka H, Pilz P, Guseo A. Primary leptomeningeal sarcomatosis. Clinicopathological report of six cases. J Neurol. 1975;211:77–93. [DOI] [PubMed] [Google Scholar]
- 18.Martin BF, Moore HC. Primary meningeal sarcomatosis. South Med J. 1970;63:419–22. [DOI] [PubMed] [Google Scholar]
- 19.Griepentrog F. [Diffuse meningeal sarcomatosis]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1952;188:549–55. [DOI] [PubMed] [Google Scholar]
- 20.Tamanini JVG, et al. Primary sarcoma of the leptomeninges: unusual presentation and previously undescribed neuroimaging features. Arq Neuropsiquiatr. 2019;77:675–6. [DOI] [PubMed] [Google Scholar]
- 21.Nair R, Nayal B, Beedkar S, Menon G. A tumour registry initiative. World Neurosurg X. 2023;20: 100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen-qing H, et al. Statistical analysis of central nervous system tumors in China. J Neurosurg. 1982;56:555–64. [DOI] [PubMed] [Google Scholar]
- 23.Habib A, et al. Soft tissue sarcomas of the head and neck region with skull base/intracranial invasion: review of surgical outcomes and multimodal treatment strategies: a retrospective case series. Curr Oncol. 2022;29:6540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin HK, et al. Risk factors for High-Grade meningioma in brain and spine: systematic review and Meta-analysis. World Neurosurg. 2021;151:e718–30. [DOI] [PubMed] [Google Scholar]
- 25.Ladra MM, et al. Local failure in parameningeal rhabdomyosarcoma correlates with poor response to induction chemotherapy. Int J Radiat Oncol Biol Phys. 2015;92:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset from the SEER database that was generated and/or analyzed during the current study is available in the SEER dataset repository (https://seer.cancer.gov/). The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.