Abstract
Background
Existing evidence highlights associations between sleep behaviors and dementia risk; however, the impact of adhering to a healthy sleep pattern on dementia risk remains unclear.
Methods
Of 406,364 UK Biobank participants aged 40–64, we excluded those who had withdrawn, had incomplete sleep data, or had dementia at baseline, yielding a final sample of 333,014. Participants were enrolled between 2006 and 2010, with follow-up extending from recruitment to dementia diagnosis, death, loss to follow-up, or the censoring date (December 2022), whichever came first. Incident dementia was identified using hospital inpatient and death records, along with primary care data, with cases diagnosed at a mean age of 70.0 years (standard deviation [SD]: 5.6). Sleep-related questionnaire items from the UK Biobank were summarized into five sleep behaviors: sleeping 7–8 h daily, early chronotype, absence of frequent insomnia, no snoring, and no frequent daytime sleepiness. Each behavior meeting the healthy criterion was assigned one point, resulting in a total range from 0 to 5, with higher scores indicating better adherence to a healthy sleep pattern. Cox proportional hazards models were used to assess the association between healthy sleep patterns and dementia risk, adjusting for demographics, lifestyle factors, and medical history. A subset of 33,401 participants underwent brain magnetic resonance imaging (MRI) scans during the 9.4-year median period between sleep assessment and imaging. The imaging analysis included total brain volume, gray matter volume, white matter volume, hippocampal volume, and white matter hyperintensities (WMH).
Results
During a median follow-up of 13.8 years, 3,035 incident dementia cases were recorded, including 1,304 Alzheimer’s disease (AD) cases and 597 vascular dementia (VD) cases. A higher adherence to a healthy sleep pattern was associated with a lower dementia risk. Each 1-point increase in the healthy sleep score corresponded to a 7% reduction in dementia risk (Hazard Ratio [HR] = 0.93, 95% Confidence Interval [CI]: 0.89−0.96). Compared to participants with a score of 0−1, those with a score of 5 had a significantly lower risk of dementia (HR = 0.75, 95% CI: 0.61−0.92). Benefits were more pronounced in adults aged 40–55 years than those aged 56–64 years (p for interaction < 0.001). Adherence to a healthy sleep pattern was associated with increased grey matter volume and decreased WMH volume (all p < 0.05). Mediation analysis indicates that preserving grey and white matter integrity partially mediated the dementia-risk-lowering benefit (p < 0.05).
Conclusions
Adherence to a healthy sleep pattern is associated with both a reduced risk of dementia and greater white matter integrity, underscoring the role of improving overall sleep behaviors to support brain structure and lower dementia risk.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-025-01864-x.
Keywords: Healthy sleep pattern, Dementia risk, Brain volumetric measures, Cohort study
Introduction
Dementia stands as a prevalent and significant global public health concern, impacting millions of individuals [1]. The worldwide prevalence of dementia was approximately 55.2 million in 2019, with projections indicating a threefold increase by 2050 [2]. Evidence underscores the potential for early preventive measures targeting risk factors to mitigate the onset of dementia in the population [3]. Recent studies indicate that various abnormal sleep behaviors manifest prior to dementia onset, and disturbances in sleep are associated with cognitive decline [4, 5]. Approximately 70% of individuals in the early stages of dementia experience sleep disturbances [6], making these modifiable risk factors a focal point for our hypothesis: adherence to a healthy sleep pattern may serve as a preventive measure against dementia.
From a physiological perspective, sleep is posited as a foundational process supporting memory formation, and inadequate sleep duration can compromise memory effectiveness in adults [7, 8]. Epidemiological evidence suggests that several abnormal sleep behaviors, including shorter or longer sleeping time [9–12], late chronotype [13], insomnia [7, 14], snoring [7, 15], and extreme daytime sleepiness [16–18], are linked to an increased risk of dementia. Importantly, these abnormal sleep behaviors tend to cluster among individuals, with their combination indicating the severity of sleep disturbances [19, 20]. Thus, a comprehensive assessment of multiple sleep behaviors is crucial for elucidating their collective impact on dementia risk. Studies of individual sleep behaviors and brain volumetric measures have provided insights into the association between adherence to a healthy sleep pattern and brain structure. Both short and long sleep duration have been linked to reduced brain volume and impaired white matter integrity [21, 22]. Conversely, individuals who reported habitual sleep durations of six to eight hours per night exhibited significantly greater grey matter volume in 46 of 139 brain regions, including the hippocampi and orbitofrontal cortex [23]. Difficulty waking up and frequent daytime napping have been correlated with diminished white matter tract integrity, smaller cortical surface area and volume, and reduced cerebellar volume [24].
In our current study, we investigated the relationship between adherence to a healthy sleep pattern and the incidence of dementia by analyzing data from middle-aged adults in the UK Biobank [25–29]. Specifically, a composite score reflecting five commonly reported healthy sleep behaviors — such as sleeping 7–8 h/day, being a morning person, experiencing no frequent insomnia, no snoring, and no frequent daytime sleepiness — was employed to estimate a healthy sleep pattern. Subsequently, subgroup analyses were conducted to identify populations most likely to derive benefits from adhering to a healthy sleep pattern. Additionally, neuroimaging data were analyzed to ascertain whether a healthy sleep pattern is associated with gray and white matter from dementia-related changes.
Method
Design
Participants were sourced from the UK Biobank, a prospective cohort study encompassing more than 0.5 million recruited participants aged 37–73 years old between 2006 and 2010. From 2014 to 2020, a sub-sample of 42,806 participants underwent brain magnetic resonance imaging (MRI) scans [30]. Detailed information about the study design has been previously outlined [31]. In brief, participants contributed a diverse array of health-related data through baseline questionnaires, interviews, and physical measurements. Some participants were later invited for brain imaging. Ethical approval for the study was granted by the North West Multi-Centre Research Ethics Committee (reference: 11/NW/03820), and all participants provided informed consent prior to study enrollment. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study population
This study included participants aged 40 to 64 years (n = 406,364) at the baseline assessment. Exclusion criteria included individuals who withdrawn from the UK Biobank (n = 109), those without sleep behavior information (n = 73,142), and those with dementia at baseline (n = 99), yielded a final sample of 333,014 participants (mean [standard deviation; SD] baseline age: 54.1 [7.0] years). The brain MRI subsample included 33,401 participants (mean [SD] age: 62.8 [6.9] years), reflecting individuals with available data. During a median follow-up of 13.8 years, 3,035 incident dementia cases (mean [SD] age: 70.0 [5.6] years) were identified (see Fig. 1).
Fig. 1.
Flowchart for the selection of the study population from the UK Biobank study.
Sleep behaviors
The UK Biobank questionnaire includes seven items directly related to sleep: (1) sleep duration, (2) getting up in morning, (3) Morning/evening person (chronotype), (4) daytime napping, (5) sleeplessness/insomnia, (6) snoring, and (7) daytime dozing/sleeping. Sleep duration of participants was assessed by inquiring, “About how many hours sleep do you get in every 24 hours (please include naps)?” Getting up in morning was evaluated via the question, “On an average day, how easy do you find getting up in the morning?” to which participants could respond not at all easy, not very easy, fairly easy, very easy, do not know, or prefer not to answer. Information regarding chronotype was obtained by posing the question, “Do you consider yourself to be?” with response categories including definitely a morning person, more a morning than an evening person, more an evening than a morning person, definitely an evening person, do not know, and prefer not to answer. Nap during day was assessed by asking, “Do you have a nap during the day?” with response options of never/rarely, sometimes, usually, or prefer not to answer. “Do you have a nap during the day?” Response categories were never/rarely, sometimes, usually, prefer not to answer. Insomnia was evaluated through the question, “Do you have trouble falling asleep at night or do you wake up in the middle of the night?” Participants could select answers from never/rarely, sometimes, usually, and prefer not to answer. Snoring was appraised by inquiring, “Does your partner or a close relative or friend complain about your snoring?” Response options included yes, no, do not know, and prefer not to answer. Lastly, daytime sleeping was assessed through the question, “How likely are you to doze off or fall asleep during the daytime when you don’t mean to (e.g., when working, reading, or driving)?” Response categories were never/rarely, sometimes, often, do not know, prefer not to answer, and all of the time.
Based on previous literature [25, 32–35], we selected five behaviors—sleep duration, chronotype, insomnia, snoring, and daytime sleepiness—to construct a healthy sleep score. Healthy sleep behaviors were defined as: sleeping 7–8 h per night; having an early chronotype (morning or more morning than evening); no snoring; no frequent insomnia (never/rarely or sometimes); and no frequent daytime sleepiness (never/rarely or sometimes). One point was assigned for each healthy sleep behavior, with a total score ranging from 0 to 5. Higher scores indicated greater adherence to a healthy sleep pattern. This scoring system has also been validated in the China Kadoorie Biobank study [32, 36].
Covariates
In our study, covariate information encompassed age, sex, ethnicity, education level, socioeconomic status, smoking status, alcohol intake, physical activity, healthy diet score, body mass index (BMI), hypertension status, diabetes status, high cholesterol status, cardiovascular disease status, and apolipoprotein E (APOE) e4 allele status.
Ethnicity was categorized as White or non-White, which included individuals identifying as Asian, Black, or other racial backgrounds [37]. Education level was classified into three categories: high, which included participants with a college or university degree; intermediate, which encompassed individuals with Advanced (A) or Advanced Subsidiary (AS) Level qualifications, or their equivalent such as the International Baccalaureate; and low, which included participants with none of the aforementioned qualifications [38]. Socioeconomic status was assessed using the Townsend Deprivation Index (range − 6.26−11.1), which incorporates data on social class, employment, car availability, and housing [39–41]. Participants were classified into three groups based on their scores, ranging from least deprived (-6.26 − -3.96), medium deprived (-3.95 −1.23), and most deprived (1.2 −11.1). Smoking status was categorized as never, previous, or current [38]. Alcohol intake was divided into five levels: never or on special occasions only, 1−3 times per month, 1−2 times per week, 3−4 times per week, daily or almost daily [42]. Physical activity was quantified by total metabolic equivalent task (MET) min/week [25]. The healthy diet score was determined based on intakes of vegetables, fruit, fish, unprocessed red meat, and processed meat [43]. We defined healthy diets according to the existing definition calculated on the basis of the following factors: vegetable intake of at least four tablespoons each day; fruit intake of at least three pieces each day; fish intake of at least twice each week; unprocessed red meat intake of no more than twice each week; and processed meat intake of no more than twice each week. One point was given for each favourable dietary factor and the total diet score ranged from 0 to 5. Healthy diet was categorized as poor (0−1), medium (2−3), or ideal (4−5) [43, 44]. BMI was calculated by dividing weight in kilograms by the square of height in meters, and the World Health Organization criteria were used to classify BMI into categories: underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30.0 kg/m2) [45]. Prevalent hypertension, diabetes, high cholesterol, and cardiovascular disease were categorized as present or absent at baseline, denoted as yes or no [46]. APOE allele status was based on two single nucleotide polymorphisms: rs7412 and rs429358 [38]. The number of APOE e4 alleles in each participant was categorized as none, one, or two [47]. In our multivariable-adjusted model, age and physical activity were treated as continuous variables. The following covariates were treated as categorical variables with specified reference groups: sex (women), ethnicity (White), education level (low), socioeconomic status (least deprived [-6.26 − -3.96]), smoking status (current smokers), alcohol intake (daily or almost daily), BMI (normal weight [18.5−24.9]), healthy diet score (poor [0−1 score]), hypertension status (present), diabetes status (present), high cholesterol status (present), cardiovascular disease status (present), and APOE e4 allele status (none).
Outcomes
Primary outcomes
The primary outcome was all-cause dementia, with its major subtypes, Alzheimer’s disease (AD) and vascular dementia (VD), investigated as secondary outcomes. Dementia outcomes were derived from two sources in the UK Biobank datasets: first occurrences (Category 1712), which include data on initial dementia diagnoses from hospital inpatient records, death records, and primary care records; and algorithmically defined outcomes (Category 47), identified using algorithms developed by the UK Biobank Outcome Adjudication Group, based on ICD-10 codes from hospital admissions, and death records [48]. The follow-up duration extended from the recruitment date to the date of first diagnosis, death, loss to follow-up, or the censoring date (December 2022), whichever came first.
Brain MRI acquisition
MRI data were collected from four UK Biobank imaging centers (Cheadle, Reading, Newcastle, and Bristol). Participants underwent scanning on a Siemens Skyra 3T MRI system equipped with a standard Siemens 32-channel head coil. T1-weighted imaging (resolution: 1.0 × 1.0 × 1.0 mm; field-of-view: 208 × 256 × 256 matrix) and T2 FLAIR imaging (resolution: 1.05 × 1.0 × 1.0 mm; field-of-view: 192 × 256 × 256 matrix) were used to capture brain tissue and structure volumes. Image processing was conducted via the UK Biobank’s standardized pipeline, incorporating publicly available tools, including FMRIB Software Library (FSL, version 5.0.10) and FreeSurfer (version 6.0) [49, 50].
MRI outcomes
Structural brain MRI data underwent processing, and quality checks were conducted using an automated pipeline [50]. Postprocessed measures derived from the UK Biobank, used in this study, encompassed total volume of brain, grey matter volume, white matter volume, white matter hyperintensity (WMH) volume, peri-ventricular WMH volume, deep WMH volume, and hippocampal volume. Extreme outliers beyond mean ± 4 SD were excluded. All MRI indices were standardized to z-scores. WMH volume and its peri-ventricular were log-transformed before z-standardization due to their positively-skewed distribution. Deep WMH volume was log-transformed with an offset of 1 to achieve a normal distribution. To account for individual differences in head size, grey matter volume, WMH volume, peri-ventricular WMH volume, and deep WMH volume were adjusted for total intracranial volume (TIV) using the residual method. Separate linear regression models were fitted for each MRI measure, with TIV as the independent variable. The residuals from these regression models, representing TIV-adjusted values for each neuroimaging metric, were extracted and utilized as the adjusted MRI values in subsequent analyses.
Statistical analyses
Summaries of the baseline characteristics of study participants are provided as the mean (SD) for continuous variables or number (percentage) for categorical variables. We combined participants with healthy sleep scores of 0 and 1 into one category as there were a small number of individuals. Missing values for covariates were imputed using sex-specific mean values for continuous variables and the missing indicator approach for categorical variables [26, 32]. The percentage of missing values is provided in Table S1. Baseline characteristics were also compared between individuals included and those excluded due to missing values (see Table S2).
Cox proportional hazards regression models were used to estimate hazard ratio (HR) and 95% confidence interval (CI) for the association between the healthy sleep score and dementia risk. The Schoenfeld residuals method validated the proportional-hazards assumption, revealing no violations. We constructed three models. Model 1 included only the primary exposure. Model 2 adjusted for demographic and lifestyle factors, including age at recruitment, gender, ethnicity, education level, socioeconomic status, smoking status, alcohol intake, physical activity, healthy diet score, and BMI. Model 3, the fully adjusted model, further incorporated comorbidities and genetic risk factors (hypertension, diabetes, high cholesterol, cardiovascular disease, and APOEe4 allele). Model 3 is the main model. Analyses with MRI outcomes were additionally adjusted for time between baseline examination and MRI examination. Comprehensive information on the covariates is available in the Supplementary Methods Component analysis was used to assess the associations between individual sleep behaviors and dementia, adjusting for other sleep behaviors in the fully adjusted model. This approach allowed us to evaluate the contribution of each sleep behavior to the overall relationship with dementia.
Additionally, we conducted stratified analyses, examining the interaction between the healthy sleep score and various factors. Interaction terms were fitted in the fully adjusted model. The population attributable risk percent (PAR%) was calculated to estimate the proportion of dementia that could theoretically be averted with a perfect healthy sleep pattern (scored 5).
For MRI indices, we employed linear regression models to explore associations with the healthy sleep score. In our analysis, we estimated the potential mediating effects of brain structure on the association between a healthy sleep score and dementia risk. The direct effect represents the association between a healthy sleep score and dementia without mediation, while the indirect effect represents the pathway through which brain structure mediates this association. The proportion of mediation was calculated as the indirect effect divided by the total effect (the sum of the direct and indirect effects). A total of 1,000 bootstrap samples were drawn to calculate 95% bootstrap confidence intervals. This approach has been widely used in previous studies to quantify mediation effects [51, 52].
We conducted several sensitivity analyses to affirm the robustness of our findings. First, we explored the potential impact of missing data on our results by employing multiple imputation through chained equations. This approach accounted for missing covariates, generating 20 sets of imputation data based on the proportion of observations with missing values [53]. Second, we replicated the analysis among individuals without missing values. Third, recognizing the potential association between sleep medications and dementia [54], we then excluded participants who took sleep medications (n = 103). Sleep medications encompassed clomethiazole, chlormethiazole, heminevrin, vantage pharmacy sleep aid, sonata, zaleplon, stilnoct, zolpidem, welldorm tablet, zimovanels, triazolam, trazodone, and zopiclone. Fourth, we adjustment for loneliness. Fifth, to minimize the reverse causality effect on observed associations, we also excluded participants who developed dementia within the first 2 or 4 years of follow-up in separate analyses. Sixth, we then employed the Fine and Gray sub-distribution hazard model to address the competing risk of death, analyzing the association of the healthy sleep score with dementia.
Statistical analyses were executed via software of Stata (version 17 (StataCorp, College Station, TX, USA) and R (version 4.0.0, R Foundation for Statistical Computing, Vienna, Austria). A two-sided P < 0.05 was deemed statistically significant.
Results
Participant characteristics
The study included 333,014 participants, with a mean (SD) age of 54.1 (7.0) years. Among them, 56% were women, and 24% were carriers of the APOE e4 allele. Over a median follow-up of 13.8 years, 3,035 dementia cases were documented, including 1,304 AD cases and 597 VD cases. The distribution of participants by healthy sleep scores was as follows: 8,245 (3%) scored between 0 and 1; 37,803 (11%) scored 2; 93,366 (28%) scored 3; 122,458 (37%) scored 4; and 71,142 (21%) scored 5. Higher scores reflect better adherence to a healthy sleep pattern. Notably, women and nonsmokers exhibited a higher likelihood of achieving a more favorable healthy sleep score. Participants with elevated healthy sleep scores tended to be white, highly educated, less deprived, physically active, with lower BMI, and exhibited a lower prevalence of hypertension, diabetes, high cholesterol, and cardiovascular disease (Table 1).
Table 1.
Baseline characteristics of participants from the UK biobank according to a healthy sleep pattern
| Characteristic | Healthy Sleep Pattern | ||||
|---|---|---|---|---|---|
| 0−1 | 2 | 3 | 4 | 5 | |
| No. of participants | 8245 | 37,803 | 93,366 | 122,458 | 71,142 |
| Age, mean (SD), year | 54.4 ± 6.7 | 54.4 ± 6.8 | 54.3 ± 6.9 | 54.0 ± 7.1 | 53.7 ± 7.2 |
| Women, n (%) | 4289 (52.0) | 20,143 (53.3) | 48,962 (52.4) | 67,519 (55.1) | 45,518 (64.0) |
| White descenta, n (%) | 7583 (92.0) | 35,409 (93.7) | 87,892 (94.1) | 115,602 (94.4) | 67,392 (94.7) |
| Educationb, n (%) | |||||
| High | 2073 (25.1) | 10,893 (28.8) | 30,725 (32.9) | 44,918 (36.7) | 28,068 (39.5) |
| Intermediate | 2722 (33.0) | 12,788 (33.8) | 31,798 (34.1) | 41,081 (33.6) | 23,507 (33.0) |
| Low | 3386 (41.1) | 13,802 (36.5) | 30,156 (32.3) | 35,598 (29.1) | 19,091 (26.8) |
| Socioeconomic status quintilec, n (%) | |||||
| 1 (least deprived) | 1244 (15.1) | 6702 (17.7) | 18,235 (19.5) | 25,297 (20.7) | 15,288 (21.5) |
| 2−4 | 4527 (54.9) | 21,815 (57.7) | 55,514 (59.5) | 74,028 (60.5) | 43,375 (61.0) |
| 5 (most deprived) | 2458 (29.8) | 9226 (24.4) | 19,493 (20.9) | 22,982 (18.8) | 12,382 (17.4) |
| BMId, kg/m2, n (%) | |||||
| < 18.5 | 23 (0.3) | 150 (0.4) | 415 (0.4) | 659 (0.5) | 510 (0.7) |
| ≥ 18.5 to < 25.0 | 1480 (18.0) | 9057 (24.0) | 26,957 (28.9) | 43,033 (35.1) | 31,115 (43.7) |
| ≥ 25.0 to < 30.0 | 3039 (36.9) | 15,405 (40.8) | 39,987 (42.8) | 51,686 (42.2) | 27,989 (39.3) |
| ≥ 30.0 | 3627 (44.0) | 12,956 (34.3) | 25,560 (27.4) | 26,537 (21.7) | 11,236 (15.8) |
| Smoking status, n (%) | |||||
| Never | 3568 (43.3) | 18,014 (47.7) | 48,931 (52.4) | 69,940 (57.1) | 45,190 (63.5) |
| Previous | 3036 (36.8) | 13,651 (36.1) | 32,078 (34.4) | 40,008 (32.7) | 20,818 (29.3) |
| Current | 1619 (19.6) | 6034 (16.0) | 12,094 (13.0) | 12,199 (10.0) | 4991 (7.0) |
| Alcohol intake, n (%) | |||||
| Daily or most daily | 1636 (19.8) | 8038 (21.3) | 19,743 (21.2) | 24,753 (20.2) | 12,278 (17.3) |
| 3−4 times/week | 1575 (19.1) | 8181 (21.6) | 22,333 (23.9) | 30,481 (24.9) | 17,576 (24.7) |
| 1−2 times/week | 1976 (24.0) | 9539 (25.2) | 24,155 (25.9) | 32,820 (26.8) | 19,819 (27.9) |
| 1−3 times/month | 1026 (12.4) | 4437 (11.7) | 10,521 (11.3) | 13,531 (11.1) | 8393 (11.8) |
| Never or special occasions only | 2027 (24.6) | 7579 (20.1) | 16,555 (17.7) | 20,822 (17.0) | 13,047 (18.3) |
|
Physical activity, METs (min/week), mean (SD) |
2389.0 ± 2539.2 | 2465.1 ± 2482.5 | 2570.0 ± 2491.8 | 2636.5 ± 2457.3 | 2751.5 ± 2462.3 |
| Healthy diet scoree, n (%) | |||||
| 0−1 | 1484 (18.0) | 5891 (15.6) | 12,936 (13.9) | 14,599 (11.9) | 6561 (9.2) |
| 2−3 | 4116 (49.9) | 18,808 (49.8) | 46,083 (49.4) | 59,452 (48.6) | 32,508 (45.7) |
| 4−5 | 2303 (27.9) | 11,769 (31.1) | 31,527 (33.8) | 45,209 (36.9) | 30,469 (42.8) |
| Hypertension, n (%) | 5101 (61.9) | 21,595 (57.1) | 49,989 (53.5) | 60,800 (49.7) | 31,708 (44.6) |
| Diabetes, n (%) | 1004 (12.2) | 2969 (7.9) | 5436 (5.8) | 5543 (4.5) | 2450 (3.4) |
| High cholesterol, n (%) | 3056 (37.1) | 12,016 (31.8) | 26,168 (28.0) | 30,105 (24.6) | 15,223 (21.4) |
| Cardiovascular disease, n (%) | 957 (11.6) | 3120 (8.3) | 6041 (6.5) | 6156 (5.0) | 2853 (4.0) |
| APOE e4, n (%) | |||||
| 0 | 4744 (57.5) | 22,115 (58.5) | 55,063 (59.0) | 72,895 (59.5) | 42,418 (59.6) |
| 1 | 1719 (20.9) | 7925 (21.0) | 20,085 (21.5) | 26,671 (21.8) | 15,750 (22.1) |
| 2 | 165 (2.0) | 692 (1.8) | 1814 (1.9) | 2431 (2.0) | 1448 (2.0) |
Continuous variables are presented as means (SD). Categorical variables are presented as numbers (percentages). Abbreviations: APOE, apolipoprotein E; BMI, body mass index; MET, metabolic equivalent. SD, SD, standard deviation
a Represents self-reported racial and ethnic background. b Education level was categorized into three groups: high (college/university degree), intermediate (Advanced [A] or Advanced Subsidiary [AS] Level qualifications), and low (none of the aforementioned), and low (none of the aforementioned). c Socioeconomic status was determined using the Townsend Deprivation Index, categorized into quintiles (1, 2−4, and 5). Higher scores indicate greater deprivation, reflecting factors such as social class, employment status, car availability, and housing conditions. d BMI was calculated by dividing weight (kg) by height (meters2). e The healthy diet score was based on consumption of vegetables, fruit, fish, unprocessed red meat, and processed meat. Participants were categorized as having a poor (score: 0–1), medium (score: 2–3), or ideal (score: 4–5) dietary pattern
Associations between adherence to a healthy sleep pattern and dementia risk
Participants with a healthy sleep score of 5 had a reduced risk of dementia (HR = 0.75; 95% CI: 0.61−0.92) and VD (HR = 0.55; 95% CI: 0.37−0.82) compared to those with scores of 0−1. Additionally, for each one-point increase in healthy sleep score, the risk of incident dementia decreased (HR = 0.93; 95% CI: 0.89−0.96) and the risk of VD decreased (HR = 0.88; 95% CI: 0.81−0.95). However, our data did not reveal a significant association between observing a healthy sleep pattern and the risk of AD (p > 0.05) (Table 2).
Table 2.
Associations between the adherence to a healthy sleep pattern and the incidence of dementia among UK biobank participants
| Healthy Sleep Pattern | No. of Events/Participants | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||||
| All-cause dementia | |||||||||
| 0–1 | 113/8245 | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||||
| 2 | 444/37,803 | 0.85 (0.69−1.04) | 0.112 | 0.90 (0.73−1.10) | 0.305 | 0.97 (0.79−1.19) | 0.758 | ||
| 3 | 901/93,366 | 0.69 (0.57−0.84) | < 0.001 | 0.77 (0.63−0.93) | 0.008 | 0.84 (0.69−1.03) | 0.087 | ||
| 4 | 1042/122,458 | 0.60 (0.50−0.73) | < 0.001 | 0.71 (0.58−0.86) | 0.001 | 0.80 (0.66−0.97) | 0.025 | ||
| 5 | 535/71,142 | 0.53 (0.43−0.65) | < 0.001 | 0.67 (0.54−0.82) | < 0.001 | 0.75 (0.61−0.92) | 0.006 | ||
| Per 1 point | 0.86 (0.83−0.89) | < 0.001 | 0.91 (0.88−0.94) | < 0.001 | 0.93 (0.89−0.96) | < 0.001 | |||
| Vascular dementia | |||||||||
| 0–1 | 35/8245 | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||||
| 2 | 102/37,803 | 0.63 (0.43−0.92) | 0.017 | 0.71 (0.48−1.04) | 0.082 | 0.83 (0.57−1.22) | 0.350 | ||
| 3 | 178/93,366 | 0.44 (0.31−0.63) | < 0.001 | 0.54 (0.38−0.78) | 0.001 | 0.67 (0.47−0.97) | 0.033 | ||
| 4 | 198/122,458 | 0.37 (0.26–0.53) | < 0.001 | 0.50 (0.35− 0.72) | < 0.001 | 0.65 (0.45−0.94) | 0.023 | ||
| 5 | 84/71,142 | 0.27 (0.18−0.40) | < 0.001 | 0.42 (0.28−0.63) | < 0.001 | 0.55 (0.37−0.82) | 0.004 | ||
| Per 1 point | 0.75 (0.70−0.81) | < 0.001 | 0.83 (0.77− 0.90) | < 0.001 | 0.88 (0.81−0.95) | 0.001 | |||
| Alzheimer’s disease | |||||||||
| 0−1 | 34/8245 | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||||
| 2 | 157/37,803 | 0.99 (0.69−1.44) | 0.977 | 1.01 (0.70−1.47) | 0.953 | 1.06 (0.73−1.54) | 0.743 | ||
| 3 | 373/93,366 | 0.95 (0.67−1.35) | 0.768 | 0.99 (0.70−1.41) | 0.960 | 1.05 (0.74−1.50) | 0.775 | ||
| 4 | 468/122,458 | 0.90 (0.64−1.27) | 0.559 | 0.98 (0.69−1.39) | 0.907 | 1.06 (0.75−1.50) | 0.751 | ||
| 5 | 272/71,142 | 0.90 (0.63−1.28) | 0.550 | 1.00 (0.70−1.43) | 0.999 | 1.08 (0.75−1.55) | 0.675 | ||
| Per 1 point | 0.97 (0.92−1.02) | 0.197 | 1.00 (0.94−1.05) | 0.885 | 1.01 (0.96−1.06) | 0.746 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio
Model 1: unadjusted; Model 2: adjusted by combining age, sex, ethnicity, education, socioeconomic status, smoking status, alcohol intake, physical activity, healthy diet score, and BMI; Model 3: based on Model 2, further combined with hypertension status, diabetes status, high cholesterol status, cardiovascular disease status, and APOE allele status
The associations between each binary (low risk vs. high risk) component of a healthy sleep pattern and the risk of incident dementia are presented in Fig. 2. Component analysis indicated that early chronotype was independently associated with a 9% reduced dementia risk, sleeping 7–8 h per day was associated with a 17% reduced risk, and no frequent daytime sleepiness was associated with a 30% reduced risk (Fig. 2). Both shorter and longer sleep durations were associated with an increased risk of dementia compared to sleeping 7–8 h per day (Sleep < 7 h/d: HR = 1.13; 95% CI: 1.04−1.24; Sleep > 8 h/d: HR = 1.41; 95% CI: 1.26−1.58; Table S3).
Fig. 2.
Associations between individual sleep behaviors and incident dementia among UK Biobank participants. Each individual component was modeled as a binary variable: sleeping 7-8 hours/day vs. sleeping more or less than 7-8 hours (reference), early chronotype vs. late chronotype (reference), no frequent insomnia vs. frequent insomnia (reference), no snoring vs. snoring (reference), no frequent daytime sleepiness vs. frequent daytime sleepiness (reference). All of the 5 individual components were included in the model simultaneously. The model was adjusted for age, sex, ethnicity, education, socioeconomic status, smoking status, alcohol intake, physical activity, healthy diet score, BMI, hypertension status, diabetes status, high cholesterol status, cardiovascular disease status, and APOE allele status.
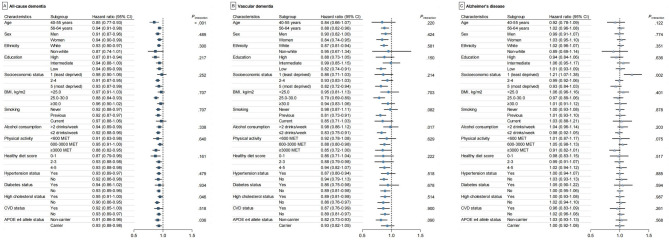
Furthermore, we observed that the effect size of the association between the healthy sleep score and incident dementia was more pronounced among younger participants (40–55 years), participants without high cholesterol, and participants without APOE 4 allele carriers compared to older participants (56–64 years), those with high cholesterol, and APOE 4 allele carriers (pinteraction < 0.05 for all) (Fig. 3). We further explored the cumulative incidence of dementia across two age groups (40–55 years and 56–64 years) and observed a consistent trend. In the 40–55 years age group, participants who scored 5 points for healthy sleep exhibited a lower incidence of dementia (15-year cumulative incidence = 0.17%, 95% CI: 0.12–0.25) compared to those with scores of 0–1 (15-year cumulative incidence = 0.72%, 95% CI: 0.49–1.05). A similar pattern was observed in the 56–64 years group, where participants with a score of 5 for healthy sleep had a lower incidence of dementia (15-year cumulative incidence = 1.78%, 95% CI: 1.62–1.96) compared to those with scores of 0–1 (15-year cumulative incidence = 2.63%, 95% CI: 2.10–3.28; Table S4, Fig. S1). Additionally, an estimated 9% of incident dementia cases (PAR% = 9%; 95% CI: 2–16%) and 18% of VD cases (PAR% = 18%; 95% CI: 1–33%) could have been prevented if all participants had adhered to the healthiest sleep score of 5 (Fig. S2).
Fig. 3.
Stratified Analyses of the Associations of the Adherence to a Healthy Sleep Pattern with Risk of Dementia. Abbreviations: APOE, apolipoprotein E; BMI, body mass index; CVD, cardiovascular disease; MET, metabolic equivalent. HR, hazard ratio; Model was adjusted for age, sex, ethnicity, education, socioeconomic status, smoking status, alcohol intake, physical activity, healthy diet score, BMI, hypertension status, diabetes status, high cholesterol status, cardiovascular disease status, and APOE allele status, except where an adjusting variable was itself tested.
Associations between adherence to a healthy sleep pattern and MRI outcomes
Adherence to a healthy sleep pattern was positively associated with grey matter volume, with each 1-point increase in the healthy sleep score associated with a 0.01 (95% CI, 0–0.02) increase in grey matter volume. Conversely, a higher healthy sleep score was negatively associated with WMH volume. Specifically, each 1-point increase in the healthy sleep score was associated with a -0.02 (95% CI, -0.03 – -0.01) reduction in overall WMH. Regionally, this reduction was seen in both periventricular WMH (-0.02; 95% CI, -0.03 – -0.01) and deep WMH volume (-0.01; 95% CI, -0.02–0) (Fig. 4.) Mediation analysis revealed that these structural brain changes partially mediated the association between healthy sleep patterns and dementia risk. Grey matter volume accounted for 1% of the mediation, overall WMH volume 3%, and periventricular WMH volume 4% (Table 3).
Fig. 4.
Association of the adherence to a healthy sleep pattern with brain structure in participants from the UK Biobank. Abbreviations: CI, confidence interval Model 1: unadjusted; Model 2: adjusted by combining age, sex, ethnicity, education, socioeconomic status, smoking status, alcohol intake, physical activity, healthy diet score, and BMI; Model 3: based on Model 2, further combined with hypertension status, diabetes status, high cholesterol status, cardiovascular disease status, and APOE allele status, time between baseline examination and MRI examination
Table 3.
Mediation analysis of brain structure in the association of adherence to a healthy seep pattern with dementia
| Total Effect | Direct Effect | Indirect Effect | Proportion Mediated | |||||
|---|---|---|---|---|---|---|---|---|
| Estimation% (95% CI) | p-value | Estimation% (95% CI) | p-value | Estimation% (95% CI) | p-value | Estimation (95% CI) | p-value | |
| Sleep → grey matter volume → dementia |
-0.25 (-0.57 − -0.03) |
0.022 |
-0.24 (-0.56 − -0.03) |
0.022 |
-0.01 (-0.02 − 0) |
0.010 |
0.96% (0.19% − 11.74%) |
0.032 |
| Sleep → WMH volume → dementia |
-0.24 (-0.55 − -0.03) |
0.020 |
-0.23 (-0.54 − -0.02) |
0.030 |
-0.01 (-0.02 − 0) |
0.006 |
3.15% (0.48% − 13.68%) |
0.026 |
| Sleep → periventricular WMH volume → dementia |
-0.25 (-0.55 − -0.03) |
0.020 |
-0.23 (-0.54 − -0.02) |
0.032 |
-0.01 (-0.02 − 0) |
< 0.001 |
4.23% (1.01% − 18.22%) |
0.020 |
| Sleep → deep WMH volume → dementia |
-0.25 (-0.55 − -0.03) |
0.020 |
-0.24 (-0.55 − -0.03) |
0.020 |
0 (-0.01 − 0) |
0.570 |
0.41% (-1.35% − 3.36%) |
0.574 |
Abbreviations: CI, confidence interval; WMH, white matter hyperintensity. Model was adjusted for age, sex, ethnicity, education, socioeconomic status, smoking status, alcohol intake, physical activity, healthy diet score, BMI, hypertension status, diabetes status, high cholesterol status, cardiovascular disease status, and APOE allele status. Arrows indicate pathways. Dementia cases in these analyses occurred after imaging (n = 49)
Sensitivity analyses
The robustness of the association between adherence to a healthy sleep pattern and incident dementia risk was further confirmed through several sensitivity analyses. Multiple imputations by chained equations yielded a similar association (HR = 0.92; 95% CI: 0.89−0.96; Table S5), as did analyses excluding participants with missing data (HR = 0.93; 95% CI: 0.89−0.97; Table S6). Excluding participants taking sleep medications (HR = 0.93; 95% CI: 0.90−0.96; Table S7) and adjusting for loneliness (HR = 0.94; 95% CI: 0.91−0.97; Table S8) had minimal impact on the observed associations. The exclusion of participants who developed dementia within the first two (HR = 0.93; 95% CI: 0.89−0.96; Table S9) and four years (HR = 0.93; 95% CI: 0.89−0.96; Table S10) of follow-up further supported the absence of reverse causation. A consistent trend was observed across different follow-up periods, with each 1-point increase in healthy sleep score associated with a reduced risk of dementia (0−5 years: HR = 0.95; 95% CI: 0.84−1.06; 6−10 years: HR = 0.97; 95% CI: 0.91−1.02; >10 years: HR = 0.93; 95% CI: 0.89−0.97; Table S11). Finally, competing risk analyses using the Fine and Gray model, which accounted for death as a competing risk, yielded similar results (HR = 0.93; 95% CI: 0.90−0.96; Table S12).
Discussion
In the current investigation, we have identified a significant association between adherence to a healthy sleep pattern and a reduced risk of incident dementia in middle-aged adults. Specifically, each 1-point increase in the score on a healthy sleep pattern correlates with a 7% decrease in the risk of dementia. Notably, the advantageous impact of adhering to a healthy sleep pattern is more pronounced in adults aged 40–55 years compared to those aged 56–64 years. Furthermore, our study reveals that the preservation of grey and white matter integrity partially mediates the risk-lowering effect of adhering to a healthy sleep pattern for dementia. Collectively, our findings strongly suggest that maintaining a healthy sleep pattern, particularly at earlier ages, confers benefits to middle-aged adults by mitigating the risk of dementia.
Several aberrant sleep behaviors have been identified as risk factors for incident dementias in longitudinal analyses. A meta-analysis of 31 longitudinal studies found that short sleep duration (≤ 6 h/night) was associated with a 46% increased risk of future dementia, whereas long sleep duration (≥ 9 h/night) was associated with a 120% increased risk [55]. Circadian rhythm disruptions, including circadian syndrome, were associated with a 40.1% increased risk of all-cause dementia [56], and impaired 24-hour rhythm integrity increased the risk of AD by 25% [57]. Frequent daytime napping was associated with a 1.27-fold increased risk of progressing from atrial fibrillation to comorbid dementia [58]. Additionally, habitual snoring was linked to an 11% increased dementia risk, even after adjusting for cardiovascular risk factors [59]. Building on these findings, our study contributes a novel approach by analyzing a composite healthy sleep pattern, incorporating five specific sleep behaviors. This multidimensional assessment provides a more comprehensive understanding of how different sleep behaviors collectively influence dementia risk. Our results demonstrate that adherence to a healthy sleep pattern is significantly associated with a lower dementia risk, emphasizing the importance of considering sleep as a whole rather than in isolated components. These findings contribute valuable insights for prevention strategies and public health interventions. Moreover, we observed that individuals aged 40–55 years derive more substantial benefits from a healthy sleep pattern compared to those aged 56–64 years. These findings align with the concept that early prevention of risk factors can effectively safeguard individual sat risk of dementia [60]. Rigorous randomized controlled trials are imperative to further ascertain the advantageous effects of a healthy sleep pattern in lowering dementia risk.
Notably, our investigation revealed differential effects of a healthy sleep pattern on the risk of various dementia subtypes, specifically AD and VD. Our study demonstrates that adhering to a healthy sleep pattern is linked to a reduced risk of VD, but not AD. Previous studies have highlighted the variation in dementia risk associated with different sleep behaviors across distinct dementia subtypes. A longitudinal study spanning a median of 12 follow-up years reveals that excessive daytime sleepiness confers varying magnitudes of risk for dementia subtypes, with a 21% increased risk for incident all-cause dementia and a significant 58% increase for VD; no discernible association between excessive daytime sleepiness and AD risk has been detected [18]. A greater incidence rate ratio of insomnia was observed in the VD population compared to the AD population [61]. Another large-scale longitudinal study of 502,383 participants reported that daytime dozing was significantly associated with an increased risk of VD, whereas no statistically significant association was found with AD [59]. These findings suggest that distinct sleep behaviors may contribute differently to the pathogenesis of various dementia subtypes. Growing evidence supports the association between sleep disorders, disrupted sleep, and negative brain vascular outcomes, including stroke, subclinical cerebrovascular disease, and related cognitive decline [62–64].
Brain structure abnormalities have been linked to sleep behaviors in cognitively healthy older adult [65]. Shorter sleep duration has been associated with increased WMH burden [66, 67] and microstructural deterioration, such as increased mean diffusivity and reduced fractional anisotropy [68], although excessive sleep duration has also been associated with greater WMH volume [69]. Insomnia has similarly been linked to higher WMH and lower white matter integrity in some studies [70], though other reports have not found such associations [71, 72]. Chronotype-related behaviors, such as difficulty getting up in the morning, have been correlated with reduced total cortical volume [24]. Additionally, a cross-sectional study of community-dwelling older adults found that individuals with frequent snoring had larger WMH volumes [73]. “Sleep-related symptoms” such as loud snoring, daytime sleepiness, likelihood to nap, and difficulty waking have been associated with increased WMH and cortical thinning [74]. Our MRI findings indicate that a composite healthy sleep pattern is linked with greater preservation of white matter structure, an association more pronounced than that observed for grey matter volume, suggesting differential neuroanatomical pathways underlying the relationship between sleep and brain health [75]. Notably, hippocampal volume was also preserved among participants with healthy sleep behaviors. Although causality cannot be inferred, these results suggest that healthy sleep may contribute to structural brain resilience. Further studies are warranted to examine how sleep impacts specific dementia subtypes and to confirm these associations longitudinally.
The present study strongly supports the hypothesis that adhering to a healthy sleep pattern, especially at earlier ages, significantly decreases the risk of incident dementia in middle-aged adults. In light of our findings, behavioral interventions—endorsed as a first-line treatment for insomnia by The American College of Physicians—may offer a practical approach to improve sleep behaviors relevant to brain health, though their influence on fixed traits like chronotype remains uncertain [76]. Our results pinpoint three key protective sleep behaviors within this healthy sleep pattern: absence of excessive daytime sleepiness, 7–8 h of sleep duration per day, and a morning chronotype. These findings lay the foundation for precisely intervening in sleep disturbances to reduce dementia risk. Given the ease of conducting the healthy sleep pattern score for population screening and its role in guiding interventions to lower dementia risk, there is significant public health importance in recognizing a healthy sleep pattern as a monitoring and interventional target for dementia prevention.
The primary strength of this study is that it represents a large-scale prospective investigation to assess the relationship between adherence to a healthy sleep pattern and both dementia risk and neuroimaging markers. Given the complex interplay between various sleep disturbances, assessing overall sleep behaviors provides a more comprehensive understanding of their collective impact. Another key strength is the use of extensive and well-characterized data from the UK Biobank, which enabled rigorous control for potential confounders. This dataset allowed us to conduct a series of sensitivity and stratification analyses, further reinforcing the robustness of our findings. A major strength of this study is the inclusion of brain imaging data, which provides insight into how sleep behaviors may influence structural markers of brain health alongside dementia risk. This study also possesses several limitations. First, the observational nature of this study precludes the validation of a causal relationship between adherence to a healthy sleep pattern and the risk of incident dementia. To establish causation and understand specific effects, randomized controlled trials of sleep interventions targeting populations at risk of dementia are imperative. These trials can contribute to further validating whether improved sleep behaviors have the potential to lower dementia risk and whether they exert differential impacts on grey and white matter integrity. Second, because the imaging data were collected cross-sectionally, temporal relationships between sleep patterns and brain structure cannot be established.
Third limitation pertains to the absence of systematic evaluation methods for sleep behaviors. While our findings, based on the five frequently reported sleep behaviors, indicate benefits for populations at risk of dementia, it is important to acknowledge that a healthy sleep pattern extends beyond these specific behaviors. Notably, other abnormal sleep behaviors, such as long napping [71], sleep apnea [65, 72], and others [73], have been documented in populations with dementia. A comprehensive analysis of various sleep behaviors could elucidate the potential interactions among them and their collective impact on dementia risk. Fourth, dementia outcomes in this study were based on hospital inpatient and death records, as well as primary-care data, which mainly capture severe cases and may not differentiate better between specific subtypes. AD and VD may be subject to misclassification, as only slightly more than half of the cases were accurately classified as AD or VD in our study. Fifth, considering individual habits, defining an early chronotype as a “healthy sleep pattern” may not be universally applicable. The potential impact of routines better aligned with an individual’s chronotype on dementia risk warrants further investigation. Sixth, while the relative association between sleep and dementia risk is more pronounced in the younger group (40–55 years), the absolute risk difference is more substantial in older adults, suggesting that further study need to validate our finding. Seventh, it is crucial to address the limitation associated with selection bias. The predominant representation of participants of European descent in this study raises concerns about the generalizability of the results to the broader population. Additionally, participants who included in our study exhibited a higher likelihood of being female, better educated, and less deprived compared to excluded participants in the UK Biobank. Consequently, caution is warranted when extrapolating these findings to the general population.
In conclusion, adherence to a healthy sleep pattern is associated with both a lower risk of incident dementia and greater white matter integrity in middle-aged adults. These results underscore the importance of sleep behaviors in supporting cognitive and structural brain health and highlight sleep as a potential target for dementia prevention.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
.This research has been conducted using the UK Biobank Resource under Application Number 92098.
Abbreviations
- AD
Alzheimer’s disease
- APOE
Apolipoprotein E
- BMI
Body mass index
- CI
Confidence interval
- HR
Hazard ratio
- MET
Metabolic equivalent task
- MRI
Magnetic resonance imaging
- PAR
Population attributable risk percent
- WMH
White matter hyperintensity
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- VD
Vascular dementia
Author contributions
Y.T., Z.W., and T.W. contributed to the conception and design of the study. Y.T., J.C., A.L., and Y.Z. contributed to the acquisition and analysis of data. T.W., W.S., X.D.L., Y.X., and H.J.L contributed to drafting the text or preparing the figures.
Funding
This work was supported by National Key Research and Development Program of China (2022YFC3602600 and 2023YFC3603200), National Natural Science Foundation of China (82220108009 and 82401664), Beijing Outstanding Young Scientist Program (JWZQ20240101023), STI2030-Major Projects (2021ZD0201801), and Beijing Hospitals Authority Youth Programme (QML20230802).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethical approval for the study was granted by the North West Multi-Centre Research Ethics Committee (reference: 11/NW/03820), and all participants provided informed consent prior to study enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tao Wei and Jie Chang contributed equally to this work.
Contributor Information
Zhibin Wang, Email: wangzhibinxw@163.com.
Yi Tang, Email: tangyi@xwhosp.org.
References
- 1.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734. [DOI] [PubMed] [Google Scholar]
- 2.Estimation of the global prevalence of dementia. In 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. 2022;7(2):e105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66(10):1210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for alzheimer’s disease dementia. Lancet Neurol. 2019;18(3):296–306. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Wei T, Xu S, et al. Sleep disorders causally affect the brain cortical structure: A Mendelian randomization study. Sleep Med. 2023;110:243–53. [DOI] [PubMed] [Google Scholar]
- 6.Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58(3):480–6. [DOI] [PubMed] [Google Scholar]
- 7.Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. [DOI] [PubMed] [Google Scholar]
- 8.Wee N, Asplund CL, Chee MW. Sleep deprivation accelerates delay-related loss of visual short-term memories without affecting precision. Sleep. 2013;36(6):849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JC, Espeland MA, Brunner RL, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement. 2016;12(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan S, Ma W, Yang R, et al. Sleep duration, genetic susceptibility, and alzheimer’s disease: a longitudinal UK Biobank-based study. BMC Geriatr. 2022;22(1):638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavaillès C, Carrière I, Wagner M et al. Trajectories of sleep duration and timing before dementia: a 14-year follow-up study. Age Ageing 2022; 51(8). [DOI] [PubMed]
- 12.Fan L, Xu W, Cai Y, et al. Sleep duration and the risk of dementia: A systematic review and Meta-analysis of prospective cohort studies. J Am Med Dir Assoc. 2019;20(12):1480–e14871485. [DOI] [PubMed] [Google Scholar]
- 13.Thapa N, Kim B, Yang JG et al. The relationship between chronotype, physical activity and the estimated risk of dementia in Community-Dwelling older adults. Int J Environ Res Public Health 2020; 17(10). [DOI] [PMC free article] [PubMed]
- 14.de Almondes KM, Costa MV, Malloy-Diniz LF, et al. Insomnia and risk of dementia in older adults: systematic review and meta-analysis. J Psychiatr Res. 2016;77:109–15. [DOI] [PubMed] [Google Scholar]
- 15.Steiner MC, Ward MJ, Ali NJ. Dementia and snoring. Lancet. 1999;353(9148):204. [DOI] [PubMed] [Google Scholar]
- 16.Tsapanou A, Gu Y, Manly J, et al. Daytime sleepiness and sleep inadequacy as risk factors for dementia. Dement Geriatr Cogn Dis Extra. 2015;5(2):286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smagula SF, Jia Y, Chang CH, et al. Trajectories of daytime sleepiness and their associations with dementia incidence. J Sleep Res. 2020;29(6):e12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavaillès C, Berr C, Helmer C, et al. Complaints of daytime sleepiness, insomnia, hypnotic use, and risk of dementia: a prospective cohort study in the elderly. Alzheimers Res Ther. 2022;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vyazovskiy VV, Walton ME, Peirson SN, et al. Sleep homeostasis, habits and habituation. Curr Opin Neurobiol. 2017;44:202–11. [DOI] [PubMed] [Google Scholar]
- 20.Cao Z, Hou Y, Yang H, et al. Healthy sleep patterns and common mental disorders among individuals with cardiovascular disease: A prospective cohort study. J Affect Disord. 2023;338:487–94. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Li X, Wang J, et al. Association of past and current sleep duration with structural brain differences: A large population-based study from the UK biobank. Sleep Med. 2024;119:179–86. [DOI] [PubMed] [Google Scholar]
- 22.Stolicyn A, Lyall LM, Lyall DM et al. Comprehensive assessment of sleep duration, insomnia, and brain structure within the UK biobank cohort. Sleep 2024; 47(2). [DOI] [PMC free article] [PubMed]
- 23.Tai XY, Chen C, Manohar S, et al. Impact of sleep duration on executive function and brain structure. Commun Biol. 2022;5(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyall LM, Stolicyn A, Lyall DM, et al. Lifetime depression, sleep disruption and brain structure in the UK biobank cohort. J Affect Disord. 2024;374:247–57. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Zhou T, Ma H, et al. Healthy sleep patterns and risk of incident arrhythmias. J Am Coll Cardiol. 2021;78(12):1197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng T, Li X, Ma H, et al. Adherence to a healthy sleep pattern and risk of chronic kidney disease: the UK biobank study. Mayo Clin Proc. 2022;97(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan C, Ye J, Wen Y, et al. The associations between sleep behaviors, lifestyle factors, genetic risk and mental disorders: A cohort study of 402 290 UK biobank participants. Psychiatry Res. 2022;311:114488. [DOI] [PubMed] [Google Scholar]
- 28.Li ZH, Huang QM, Gao X, et al. Healthy sleep associated with lower risk of hypertension regardless of genetic risk: A Population-Based cohort study. Front Cardiovasc Med. 2021;8:769130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou T, Yuan Y, Xue Q, et al. Adherence to a healthy sleep pattern is associated with lower risks of all-cause, cardiovascular and cancer-specific mortality. J Intern Med. 2022;291(1):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Littlejohns TJ, Holliday J, Gibson LM, et al. The UK biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat Commun. 2020;11(1):2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan M, Sun D, Zhou T, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. 2020;41(11):1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou M, Lu D, Luo Z, et al. Association of healthy sleep patterns with incident sepsis: a large population-based prospective cohort study. Crit Care. 2025;29(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cong H, Li Z, Zhang J, et al. Adherence to a healthy sleep pattern and the risk of kidney stone disease: A prospective study of UK biobank. J Psychosom Res. 2025;189:111999. [DOI] [PubMed] [Google Scholar]
- 35.Ni J, Zhou Q, Meng SY, et al. Sleep patterns, physical activity, genetic susceptibility, and incident rheumatoid arthritis: a prospective cohort study. BMC Med. 2024;22(1):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong Q, Qin Z, Wang X, et al. Healthy sleep pattern reduce the risk of cardiovascular disease: A 10-year prospective cohort study. Sleep Med. 2023;105:53–60. [DOI] [PubMed] [Google Scholar]
- 37.Chang J, Liu Y, Zhao Y, et al. Association of sleep duration with excess risk of dementia among shift workers in the UK biobank: a population-based cohort study. J Neurol. 2024;271(9):6056–67. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Cao X, Li X, et al. Associations of midlife dietary patterns with incident dementia and brain structure: findings from the UK biobank study. Am J Clin Nutr. 2023;118(1):218–27. [DOI] [PubMed] [Google Scholar]
- 39.Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster HME, Celis-Morales CA, Nicholl BI, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK biobank cohort. Lancet Public Health. 2018;3(12):e576–85. [DOI] [PubMed] [Google Scholar]
- 41.Jarman B, Townsend P, Carstairs V. Deprivation indices. BMJ. 1991;303(6801):523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng Z, Lv Y, Rong S, et al. Physical frailty, genetic predisposition, and incident Parkinson disease. JAMA Neurol. 2023;80(5):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Zhou T, Song Q, et al. Ambient air pollution, healthy diet and vegetable intakes, and mortality: a prospective UK biobank study. Int J Epidemiol. 2022;51(4):1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Chang J, Zhao Y, et al. Neuroticism personality, social contact, and dementia risk: A prospective cohort study. J Affect Disord. 2024;358:391–8. [DOI] [PubMed] [Google Scholar]
- 45.Obesity. Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. [PubMed] [Google Scholar]
- 46.Ling Y, Yuan S, Huang X, et al. The association of night shift work with the risk of all-cause dementia and alzheimer’s disease: a longitudinal study of 245,570 UK biobank participants. J Neurol. 2023;270(7):3499–510. [DOI] [PubMed] [Google Scholar]
- 47.Wang HF, Zhang W, Rolls ET, et al. Hearing impairment is associated with cognitive decline, brain atrophy and Tau pathology. EBioMedicine. 2022;86:104336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson T, Schnier C, Bush K, et al. Identifying dementia outcomes in UK biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol. 2019;34(6):557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller KL, Alfaro-Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alfaro-Almagro F, Jenkinson M, Bangerter NK, et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK biobank. NeuroImage. 2018;166:400–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Z, Jiang Y, Li P, et al. Association of impaired sensitivity to thyroid hormones with hyperuricemia through obesity in the euthyroid population. J Transl Med. 2023;21(1):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui C, Liu L, Zhang T, et al. Triglyceride-glucose index, renal function and cardiovascular disease: a National cohort study. Cardiovasc Diabetol. 2023;22(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 54.Full KM, Pusalavidyasagar S, Palta P, et al. Associations of Late-Life sleep medication use with incident dementia in the atherosclerosis risk in communities study. J Gerontol Biol Sci Med Sci. 2023;78(3):438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howard C, Mukadam N, Hui EK, et al. The effects of sleep duration on the risk of dementia incidence in short and long follow-up studies: A systematic review and meta-analysis. Sleep Med. 2024;124:522–30. [DOI] [PubMed] [Google Scholar]
- 56.Yu L, Liu W, Liao C et al. The interaction between circadian syndrome and genetic susceptibility in the risk of incident dementia: A longitudinal cohort study. J Prev Alzheimers Dis 2025:100089. [DOI] [PMC free article] [PubMed]
- 57.Winer JR, Lok R, Weed L, et al. Impaired 24-h activity patterns are associated with an increased risk of alzheimer’s disease, parkinson’s disease, and cognitive decline. Alzheimers Res Ther. 2024;16(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C, He D, Yang C, et al. Daytime napping, incident atrial fibrillation, and dynamic transitions with dementia. JACC Adv. 2024;3(8):101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo C, Harshfield EL, Markus HS. Sleep characteristics and risk of stroke and dementia: an observational and Mendelian randomization study. Neurology. 2024;102(5):e209141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crouse JJ, Carpenter JS, Song YJC, et al. Circadian rhythm sleep-wake disturbances and depression in young people: implications for prevention and early intervention. Lancet Psychiatry. 2021;8(9):813–23. [DOI] [PubMed] [Google Scholar]
- 61.Baek MS, Han K, Kwon HS, et al. Risks and prognoses of alzheimer’s disease and vascular dementia in patients with insomnia: A nationwide Population-Based study. Front Neurol. 2021;12:611446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hermann DM, Bassetti CL. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology. 2016;87(13):1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Culebras A, Anwar S. Sleep apnea is a risk factor for stroke and vascular dementia. Curr Neurol Neurosci Rep. 2018;18(8):53. [DOI] [PubMed] [Google Scholar]
- 64.Gottesman RF, Lutsey PL, Benveniste H, et al. Impact of sleep disorders and disturbed sleep on brain health: A scientific statement from the American heart association. Stroke. 2024;55(3):e61–76. [DOI] [PubMed] [Google Scholar]
- 65.André C, Laniepce A, Chételat G, et al. Brain changes associated with sleep disruption in cognitively unimpaired older adults: A short review of neuroimaging studies. Ageing Res Rev. 2021;66:101252. [DOI] [PubMed] [Google Scholar]
- 66.Baril AA, Beiser AS, Mysliwiec V, et al. Slow-Wave sleep and MRI markers of brain aging in a Community-Based sample. Neurology. 2021;96(10):e1462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yaffe K, Nasrallah I, Hoang TD, et al. Sleep duration and white matter quality in Middle-Aged adults. Sleep. 2016;39(9):1743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grumbach P, Opel N, Martin S, et al. Sleep duration is associated with white matter microstructure and cognitive performance in healthy adults. Hum Brain Mapp. 2020;41(15):4397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramos AR, Dong C, Rundek T, et al. Sleep duration is associated with white matter hyperintensity volume in older adults: the Northern Manhattan study. J Sleep Res. 2014;23(5):524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang JMK, Joo SWJ, Son YDS, et al. Low white-matter integrity between the left thalamus and inferior frontal gyrus in patients with insomnia disorder. J Psychiatry Neurosci. 2018;43(6):366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li C, Schreiber J, Bittner N, et al. White matter microstructure underlies the effects of sleep quality and life stress on depression symptomatology in older adults. Front Aging Neurosci. 2020;12:578037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thurston RC, Wu M, Aizenstein HJ et al. Sleep characteristics and white matter hyperintensities among midlife women. Sleep 2020; 43(6). [DOI] [PMC free article] [PubMed]
- 73.Rostanski SK, Zimmerman ME, Schupf N, et al. Sleep disordered breathing and white matter hyperintensities in Community-Dwelling elders. Sleep. 2016;39(4):785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu J, Morys F, Dagher A, et al. Associations between sleep-related symptoms, obesity, cardiometabolic conditions, brain structural alterations and cognition in the UK biobank. Sleep Med. 2023;103:41–50. [DOI] [PubMed] [Google Scholar]
- 75.Xia Y, Shen Y, Wang Y, et al. White matter hyperintensities associated with progression of cerebral small vessel disease: a 7-year Chinese urban community study. Aging (Albany N Y). 2020;12(9):8506–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American college of physicians. Ann Intern Med. 2016;165(2):125–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.