Abstract
By reverse transcription-PCR, measles virus (MV) mRNA was detected in the brain, kidney, spleen, liver, and lung tissues obtained from 23 (45.1%) of 51 autopsy subjects, with the detection rates of each tissue ranging from 8 to 20%. Sequence analysis revealed frequent mutations in the corresponding viral protein. These results suggest that MV mutants commonly persist in apparently healthy individuals.
Measles virus (MV) is the causative agent of acute measles in young children worldwide. Although homotypic serologically, MV exhibits a considerable degree of genetic diversity and can be classified into a number of phylogenetic groups (10, 19). Besides this genetic diversity, another kind of MV mutation has been identified. Persistent infection of particular MV mutants in the brain is known to result in a rare but incurable disease called subacute sclerosing panencephalitis (SSPE) (5, 7, 20). It is generally believed that, except for the rare cases of SSPE, MV is eliminated from infected individuals by potent anti-MV immunity after recovery from acute measles. However, our previous study showed that, by using a reverse transcription (RT)-PCR method, MV mRNA was detected in 11 (18%) of 61 brain tissue samples obtained from autopsy subjects who had not exhibited SSPE-like symptoms (9). This result suggests the possibility that MV persists in apparently healthy individuals. The question of whether MV mRNA was present in human tissues other than the brain was then raised. To address this question, we obtained various tissues from autopsy subjects and tried to detect MV mRNA by RT-PCR. We report here that MV mRNA was detected not only in the brain but also in other human tissues, such as kidney, spleen, liver, and lung.
Human tissues were obtained from 51 autopsy subjects (within 24 h postmortem) during the period between April and December 1995. The autopsy subjects consisted of 33 males and 18 females, with a mean age of 54.3 years (range, 4 months to 88 years). The tissue samples were stored at −130°C until RT-PCR analysis was performed. Extraction of RNA and detection of MV nucleoprotein mRNA were performed as described previously (9). Briefly, 10 μg of total RNA extracted from the tissue samples was reverse transcribed into cDNA by using MV-specific primer NP4. The resultant cDNA was amplified by using a set of outer primers (NP1 and NP4) in the first round of PCR and a set of inner primers (NP2 and NP3) in the second round of PCR. The expected sizes of the fragments amplified by the first- and second-round PCR were 228 (nucleotides 235 to 462, including NP1 and NP4 sequences) and 149 bp (nucleotides 280 to 428, including NP2 and NP3 sequences), respectively. The PCR products were electrophoresed and visualized by UV illumination. This RT-PCR method was shown to detect MV genomic RNA of as low as 0.5 50% tissue culture-infective dose that had been mixed with MV-negative tissue samples (data not shown). Nucleotide sequences of the second-round PCR products were determined, as described previously (9, 10, 21).
Consistent with our previous results (9), 10 (19.6%) of 51 brain tissue samples were positive for MV mRNA (Table 1). Other tissues, such as lung, liver, spleen, and kidney, were also shown to be positive for MV mRNA, with detection rates of 13.7, 7.8, 10.0, and 17.6%, respectively. There was no significant difference in the MV mRNA detection rates for the five tissues. Of the 51 subjects, 15 (29.4%) had MV mRNA in only one tissue, while 8 had MV mRNA in two or more tissues. In total, 23 (45.1%) of the 51 subjects had MV mRNA in at least one tissue. Inflammatory changes were barely observed in MV mRNA-positive tissues by ordinary histophological examinations (data not shown). The cause of death of the 23 positive cases was cardiovascular disorders, such as dissecting aneurysm and myocardial infarction (12 cases), accidents (6 cases), sudden death syndrome (2 cases), chronic obstructive pulmonary disease (2 cases), and pneumonia (1 case). No significant association was observed between an MV mRNA positive result and the cause of death.
TABLE 1.
Detection of MV mRNA in autopsied human tissues
| MV-positive tissue | No. of MV-positive cases/no. of total cases (%) |
|---|---|
| Tissue type | |
| Brain | 10/51 (19.6) |
| Lung | 7/51 (13.7) |
| Liver | 4/51 (7.8) |
| Spleen | 5/50a (10.0) |
| Kidney | 9/51 (17.6) |
| No. of tissues | |
| Only one | 15/51 (29.4) |
| Two | 5/51 (9.8) |
| Three | 2/51 (3.9) |
| Four | 1/51 (2.0) |
| Total | 23/51 (45.1) |
Spleen tissue was not available for one individual who had had a splenectomy.
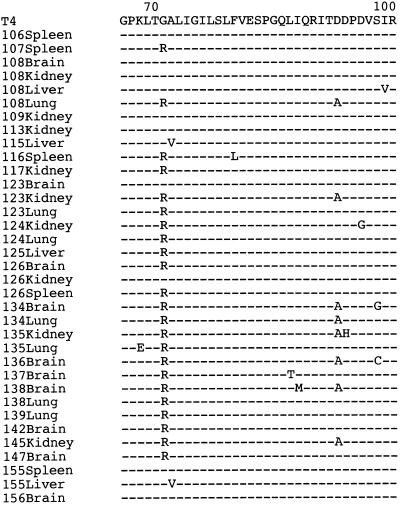
Nucleotide sequences from a total of 35 tissue-derived MV clones were determined and amino acid sequences were deduced (Fig. 1). Nine clones had the same amino acid sequence as the wild-type T4 strain did. On the other hand, the remaining 26 clones had unique amino acid sequences differing in one to three residues from that of the T4 strain. It should be noted that all 14 wild-type MV strains collected from throat swabs of typical measles patients (collected in 1995 and 1996) had the same sequence as that of T4 at both amino acid and nucleotide levels (data not shown). These results strongly suggest that the amino acid alterations observed with the 26 tissue-derived MV clones were not due to artifacts that had been caused during the RT-PCR amplification but that the mutated MV sequences were present in the tissue samples, which probably had been generated during long-term viral persistence in the tissues in the presence of anti-MV immunity. In individuals with multiple MV-positive tissues, amino acid sequences of the MV clones often varied with different tissues (Fig. 1), suggesting that different mutants could arise in an individual. This result is consistent with a previous observation that a number of different clones were detected in different areas of the brain of an SSPE patient (2).
FIG. 1.
Alignment of deduced amino acid sequences of part of the nucleoprotein sequence from tissue-derived MV and a fresh, wild-type isolate of MV. Residues identical to those of the wild-type T4 strain are indicated by dashes. Amino acid positions are depicted at the top of the figure. The virus isolate designation (the patient number and tissue) are shown to the left of the sequences. The sequences shown correspond to 7% of the entire sequence of the nucleoprotein.
Some of the human tissues were immediately minced, without being frozen, and cocultured with B95-8 cells, a cell line highly susceptible to MV field isolates (10), in an attempt to isolate infectious MV. However, infectious MV could not be isolated from the autopsied human tissues (data not shown). This result suggests the possibility that replication competence of the persisting MV has been impaired drastically due to accumulated mutations in the viral genome.
In general, replication-competent, nonmutated MV would eventually be eliminated by strong immunity against the virus after recovery from acute measles. Host immune responses are directed to various antigenic epitopes in MV proteins. As for the nucleoprotein, a number of cytotoxic T-cell epitopes and B-cell epitopes have been determined in the murine experimental system (3, 4, 6, 8). The partial nucleoprotein sequence analyzed in the present study (amino acids 66 to 100) contains one of the cytotoxic T-cell epitopes and was shown to have mutations (Fig. 1). Such mutated MV, with highly impaired replication competence, might escape from the anti-MV immune surveillance to persist in the host without causing apparent symptoms. This idea is in line with previous observations that MV mRNA and antigens were detected in certain tissues and cells, including bone marrow cells and peripheral blood mononuclear cells, obtained from healthy individuals, though at low frequencies (14, 15). If such an MV mutant regained replication competence during persistent infection, it could cause injury to infected cells through cytopathic effect of the virus and/or antiviral immune responses of the host. In fact, besides SSPE, possible associations between persistent MV infection and certain diseases, such as Crohn’s disease (16, 22, 23), otosclerosis (1, 14, 17), Paget’s disease (15, 18), epilepsy (11), aseptic chronic meningitis (13), and autoimmune hepatitis (12) have recently been documented. Further study is necessary to elucidate the mechanism and clinical significance of MV persistence in human tissues.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this study will appear in DDBJ/EMBL/GenBank Nucleotide Sequence Databases with the accession numbers AB006495 to AB006529.
Acknowledgments
This work was supported in part by a Research Program for Slow Virus Infection from the Ministry of Health and Welfare of Japan and by a research grant from Yakult Co., Ltd.
REFERENCES
- 1.Arnold W, Niedermeyer H P, Lehn N, Neubert W, Hofler H. Measles virus in otosclerosis and the specific immune response of the inner ear. Acta Otolaryngol. 1996;116:705–709. doi: 10.3109/00016489609137910. [DOI] [PubMed] [Google Scholar]
- 2.Baczko K, Lampe J, Liebert U G, Brinckmann U, ter Meulen V, Pardowitz I, Budka H, Cosby S L, Isserte S, Rima B K. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology. 1993;197:188–195. doi: 10.1006/viro.1993.1579. [DOI] [PubMed] [Google Scholar]
- 3.Beauverger P, Cardoso A I, Daviet L, Buckland R, Wild T F. Analysis of the contribution of CTL epitopes in the immunobiology of morbillivirus infection. Virology. 1996;219:133–139. doi: 10.1006/viro.1996.0230. [DOI] [PubMed] [Google Scholar]
- 4.Beauverger P, Chadwick J, Buckland R, Wild T F. Serotype-specific and canine distemper virus cross-reactive H-2Kk-restricted cytotoxic T lymphocyte epitopes in the measles virus nucleoprotein. Virology. 1994;203:172–177. doi: 10.1006/viro.1994.1470. [DOI] [PubMed] [Google Scholar]
- 5.Billeter M A, Cattaneo R, Schmid A, Eschle D, Kaelin K, Rebmann G, Udem S A, Sheppard R D, Baczko K, Liebert U G, Schneider-Schaulies S, Brinckmann U, ter Meulen V. Host and viral features in persistent measles virus infection of the brain. In: Mahy B W J, Kolakofsky D, editors. Genetics and pathogenicity of negative strand viruses. Amsterdam, The Netherlands: Elsevier/North-Holland Publishing Co.; 1989. pp. 356–366. [Google Scholar]
- 6.Buckland R, Giraudon P, Wild F. Expression of measles virus nucleoprotein in Escherichia coli: use of deletion mutants to locate the antigenic sites. J Gen Virol. 1989;70:435–441. doi: 10.1099/0022-1317-70-2-435. [DOI] [PubMed] [Google Scholar]
- 7.Cattaneo R, Schmid A, Spielhofer P, Kaelin K, Baczko K, ter Meulen V, Pardowitz J, Flanagan S, Rima B K, Udem S A, Billeter M. Mutated and hypermutated genes of persistent measles viruses which cause lethal human brain disease. Virology. 1989;173:415–425. doi: 10.1016/0042-6822(89)90554-0. [DOI] [PubMed] [Google Scholar]
- 8.Gombart A F, Hirano A, Wong T C. Conformational maturation of measles virus nucleocapsid protein. J Virol. 1993;67:4133–4141. doi: 10.1128/jvi.67.7.4133-4141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katayama Y, Hotta H, Nishimura A, Tatsuno T, Homma M. Detection of measles virus nucleoprotein mRNA in autopsied brain tissues. J Gen Virol. 1995;76:3201–3204. doi: 10.1099/0022-1317-76-12-3201. [DOI] [PubMed] [Google Scholar]
- 10.Katayama Y, Shibahara K, Kohama T, Homma M, Hotta H. Molecular epidemiology and changing distribution of genotypes of measles virus field strains in Japan. J Clin Microbiol. 1997;35:2651–2653. doi: 10.1128/jcm.35.10.2651-2653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawashima H, Miyajima T, Mori T, Yuan L, Ogihara M, Kinoue K, Takekuma K, Hoshika A. A case of intractable epilepsy positive for the detection of measles virus genome in the cerebrospinal fluid and peripheral mononuclear cells using reverse transcriptase-polymerase chain reaction. Brain Dev. 1996;18:220–223. doi: 10.1016/0387-7604(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima H, Mori T, Takekuma K, Hoshika A, Hata M, Nakayama T. Polymerase chain reaction detection of the hemagglutinin gene from an attenuated measles vaccine strain in the peripheral mononuclear cells of children with autoimmune hepatitis. Arch Virol. 1996;141:877–884. doi: 10.1007/BF01718162. [DOI] [PubMed] [Google Scholar]
- 13.Luzi P, Leoncini L, Valassina M, Federico A, Palma L. Chronic progressive leptomeningitis associated with measles virus. Lancet. 1997;350:338–339. doi: 10.1016/S0140-6736(05)63389-2. [DOI] [PubMed] [Google Scholar]
- 14.McKenna M J, Kristiansen A G, Haines J. Polymerase chain reaction amplification of a measles virus sequence from human temporal bone sections with active otosclerosis. Am J Otol. 1996;17:827–830. [PubMed] [Google Scholar]
- 15.Mills B G, Frausto A, Singer F R, Ohsaki Y, Demulder A, Roodman G D. Multinucleated cells formed in vitro from Paget’s bone marrow express viral antigens. Bone. 1994;15:443–448. doi: 10.1016/8756-3282(94)90823-0. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto H, Tanaka T, Kitamoto N, Fukuda Y, Shimoyama T. Detection of immunoreactive antigen, with a monoclonal antibody to measles virus, in tissue from a patient with Crohn’s disease. J Gastroenterol. 1995;30:28–33. doi: 10.1007/BF01211371. [DOI] [PubMed] [Google Scholar]
- 17.Niedermeyer H P, Arnold W. Otosclerosis: a measles virus associated inflammatory disease. Acta Otolaryngol. 1995;115:300–303. doi: 10.3109/00016489509139314. [DOI] [PubMed] [Google Scholar]
- 18.Reddy S V, Singer F R, Roodman G D. Bone marrow mononuclear cells from patients with Paget’s disease contain measles virus nucleocapsid messenger ribonucleic acid that has mutations in a specific region of the sequence. J Clin Endocrinol Metab. 1995;80:2108–2111. doi: 10.1210/jcem.80.7.7608263. [DOI] [PubMed] [Google Scholar]
- 19.Rota J S, Heath J L, Rota P A, King G E, Celma M L, Carabaña J, Fernandez-Muñoz R, Brown D, Jin L, Bellini W J. Molecular epidemiology of measles virus: identification of pathways of transmission and implication for measles elimination. J Infect Dis. 1996;173:32–37. doi: 10.1093/infdis/173.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Schneider-Schaulies S, Schneider-Schaulies L, Dunster L M, ter Meulen V. Measles virus expression in neural cells. In: ter Meulen V, Billeter M A, editors. Measles virus. Berlin, Germany: Springer-Verlag; 1995. pp. 101–116. [Google Scholar]
- 21.Shibahara K, Hotta H, Katayama Y, Homma M. Increased binding activity of measles virus to monkey red blood cells after long-term passage in Vero cell cultures. J Gen Virol. 1994;75:3511–3516. doi: 10.1099/0022-1317-75-12-3511. [DOI] [PubMed] [Google Scholar]
- 22.Wakefield A J, Pittilo R M, Sim R, Cosby S L, Stephenson J R, Dhillon A P, Pounder R E. Evidence of persistent measles virus infection in Crohn’s disease. J Med Virol. 1993;39:345–353. doi: 10.1002/jmv.1890390415. [DOI] [PubMed] [Google Scholar]
- 23.Wakefield A J, Sim R, Akbar A N, Pounder R E, Dhillon A P. In situ immune responses in Crohn’s disease: a comparison with acute and persistent measles virus infection. J Med Virol. 1997;51:90–100. doi: 10.1002/(sici)1096-9071(199702)51:2<90::aid-jmv2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]