Abstract
1. Junction potentials were recorded from the circular muscle cells of the guinea-pig ileum following transmural stimulation in the presence of atropine at 30 °C.
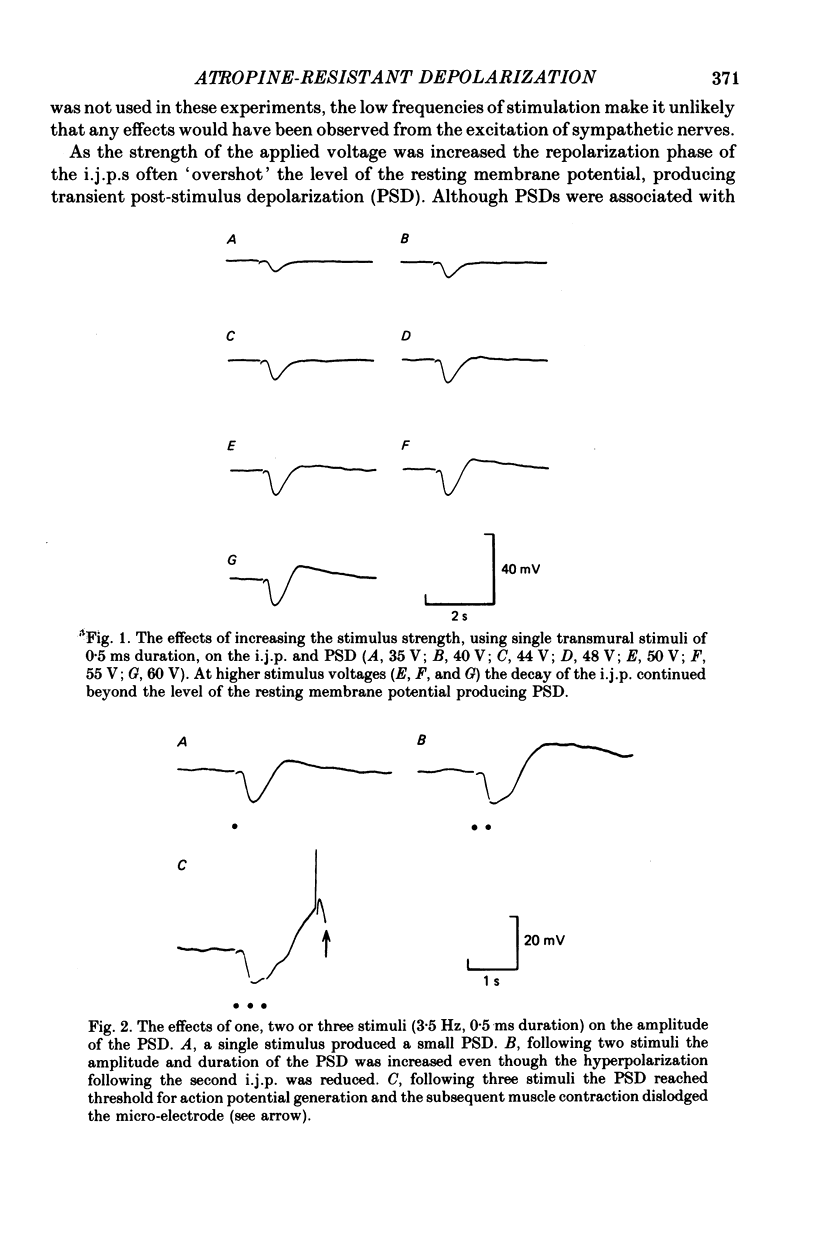
2. Single stimuli produced a transient hyperpolarization, the inhibitory junction potential (i.j.p.). At high stimulus strengths the i.j.p. was followed by a post-stimulus depolarization (PSD).
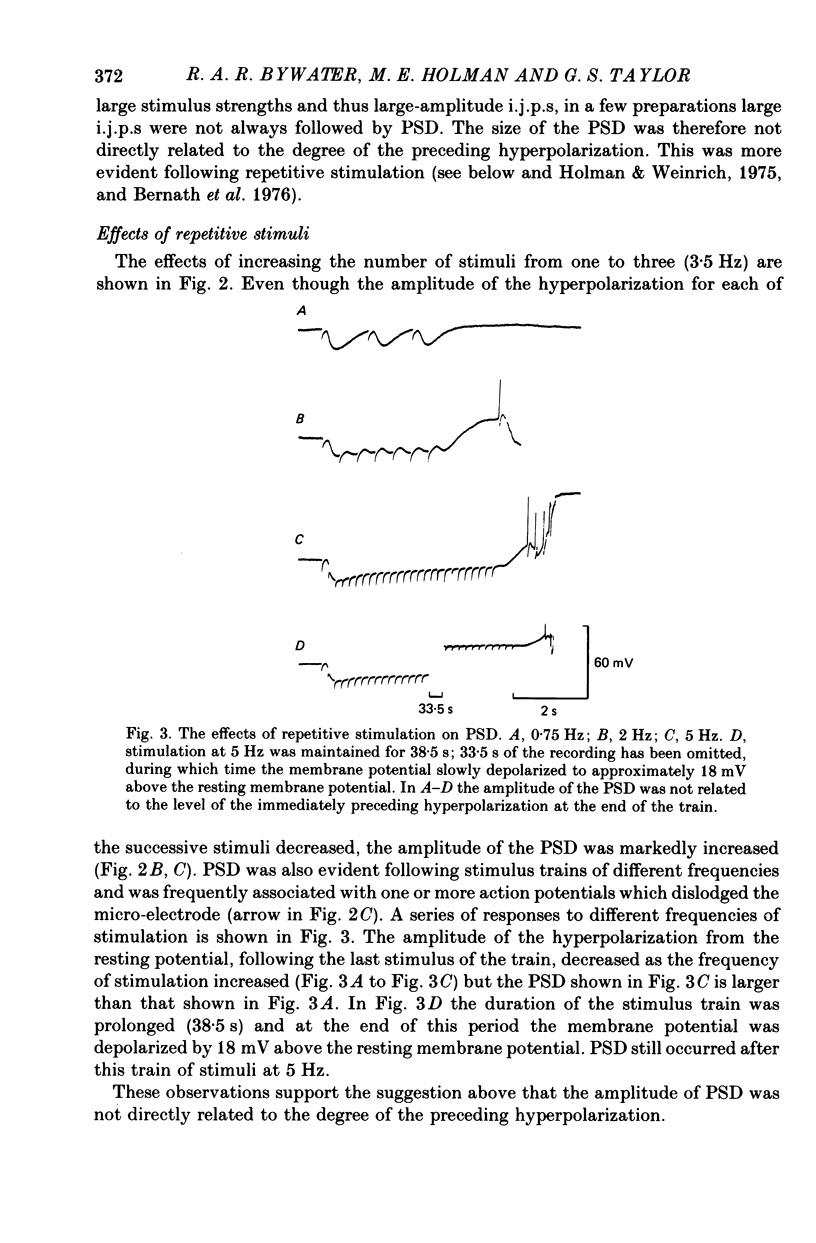
3. During repetitive stimulation the magnitude of the hyperpolarization decreased; however, at the end of the stimulus period the PSD was enhanced and often reached threshold for the generation of action potentials. Thus, the size of the PSD was not directly related to the degree of the preceding hyperpolarization.
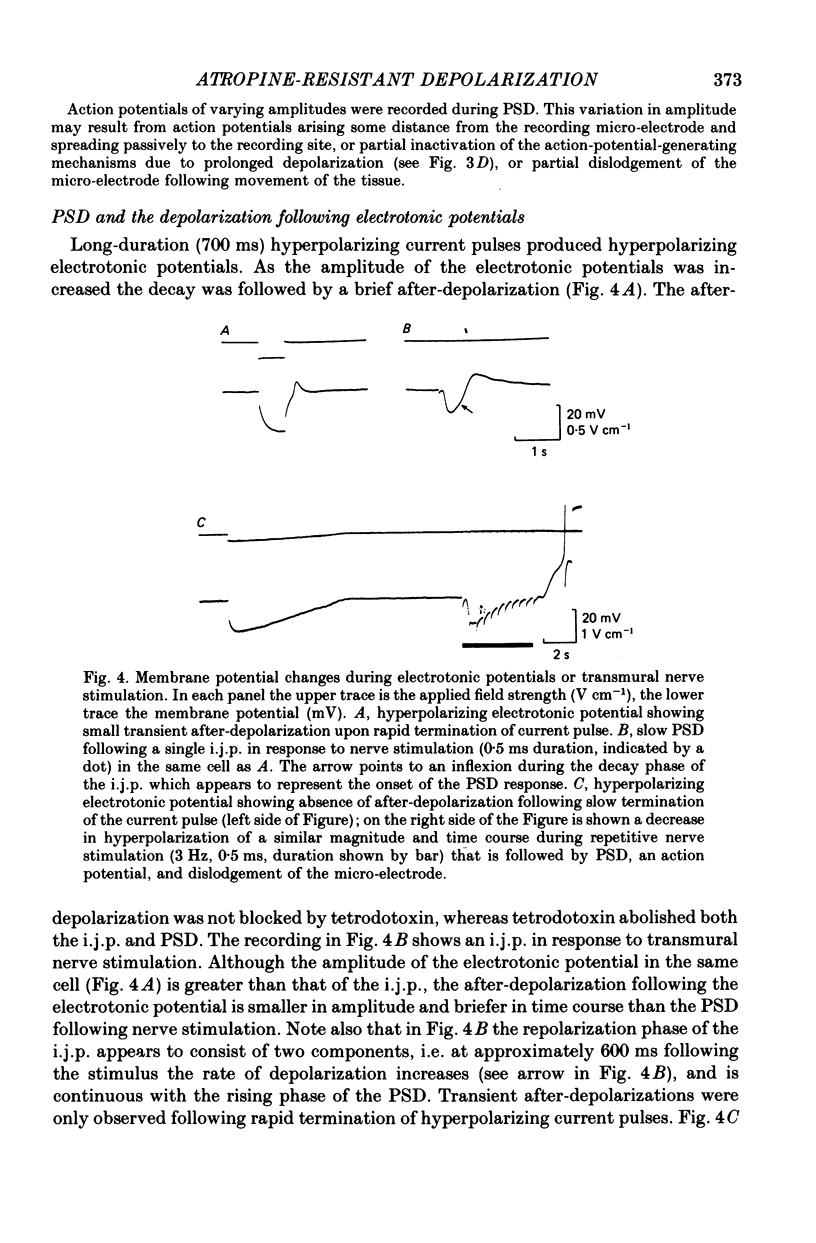
4. Hyperpolarization of the circular muscle cells was produced by the application of anodal current using large external electrodes. Rapid cessation of the applied current produced a transient after-depolarization which was shorter in time course than the PSD following the i.j.p. If the applied anodal current was reduced slowly (at a rate which mimicked the decrease in the hyperpolarization during repetitive nerve stimulation) no after-depolarization was observed.
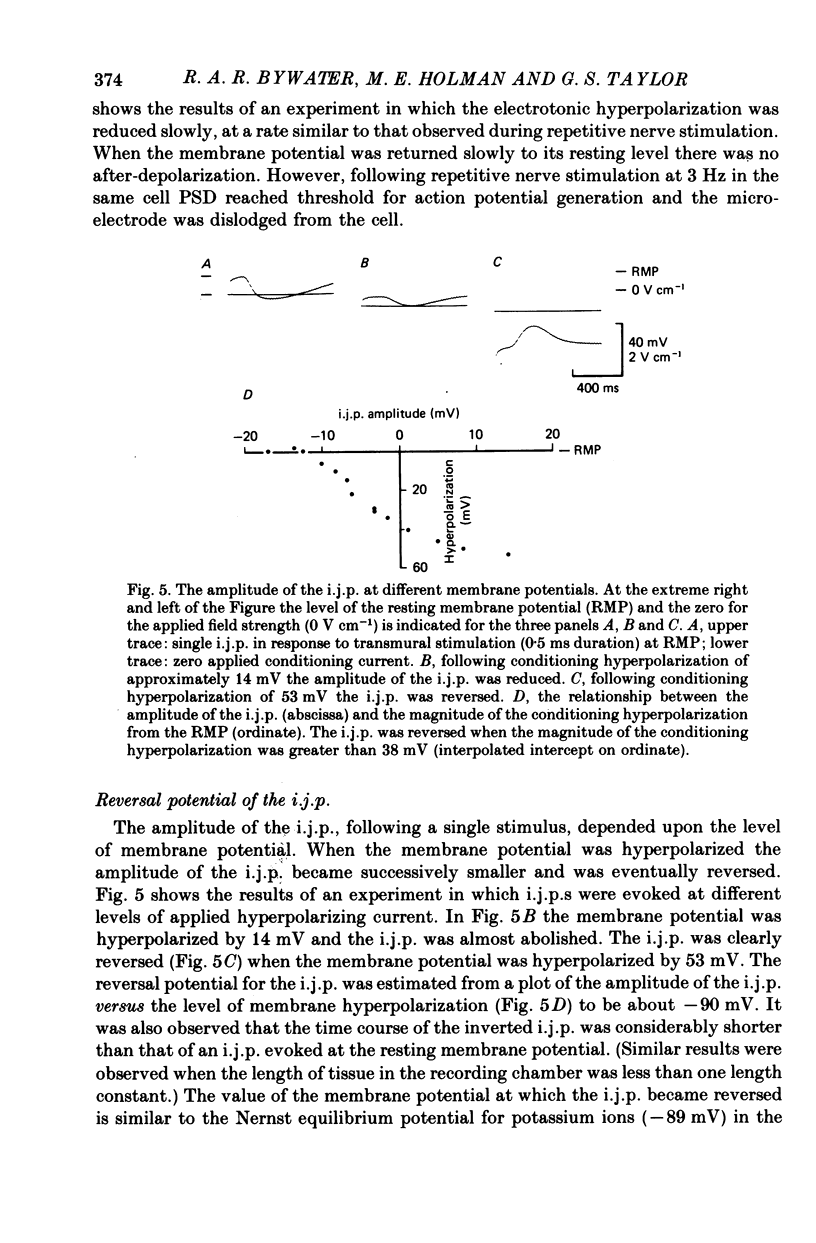
5. Conditioning hyperpolarization of the circular muscle cells reduced the amplitude of the i.j.p. The i.j.p. was reversed at membrane potentials greater than approximately -90 mV.
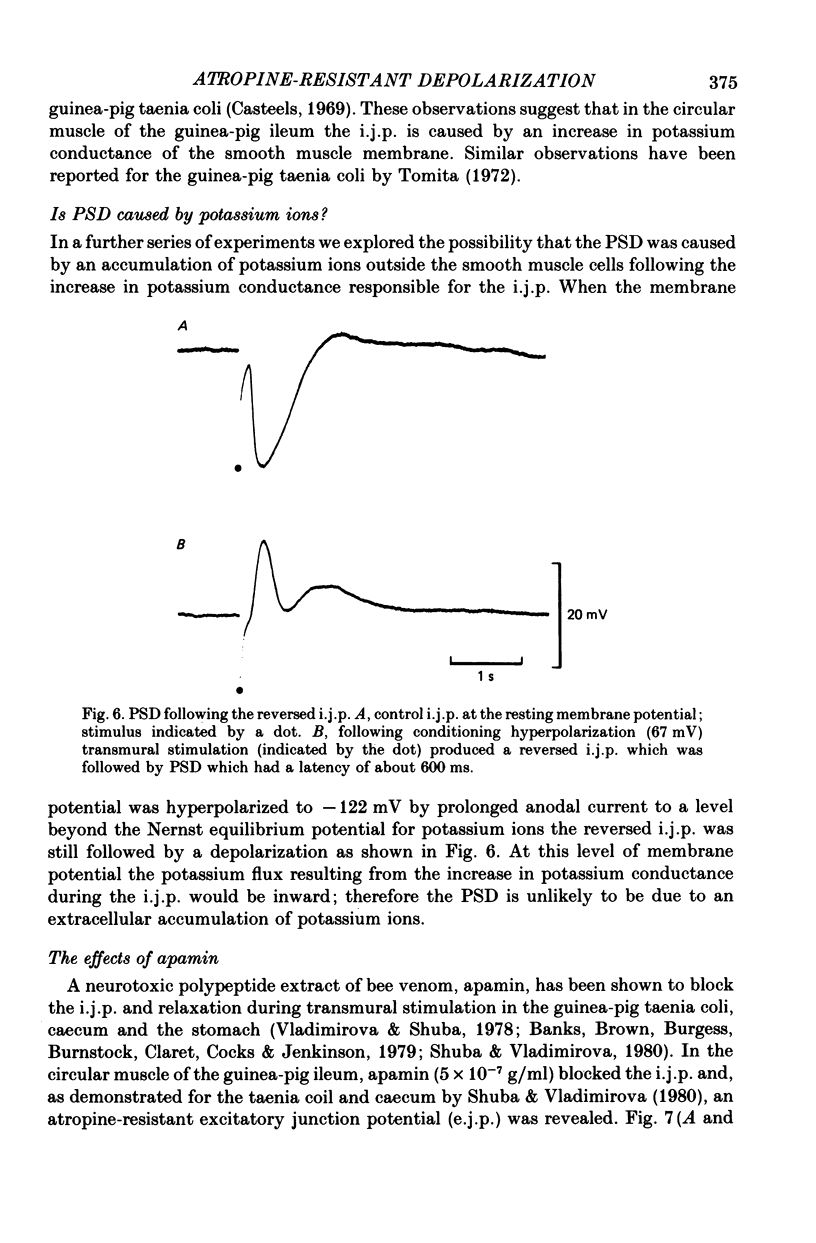
6. The PSD did not appear to be due to the extracellular accumulation of potassium ions following the i.j.p. since the PSD persisted even when the i.j.p. was reversed.
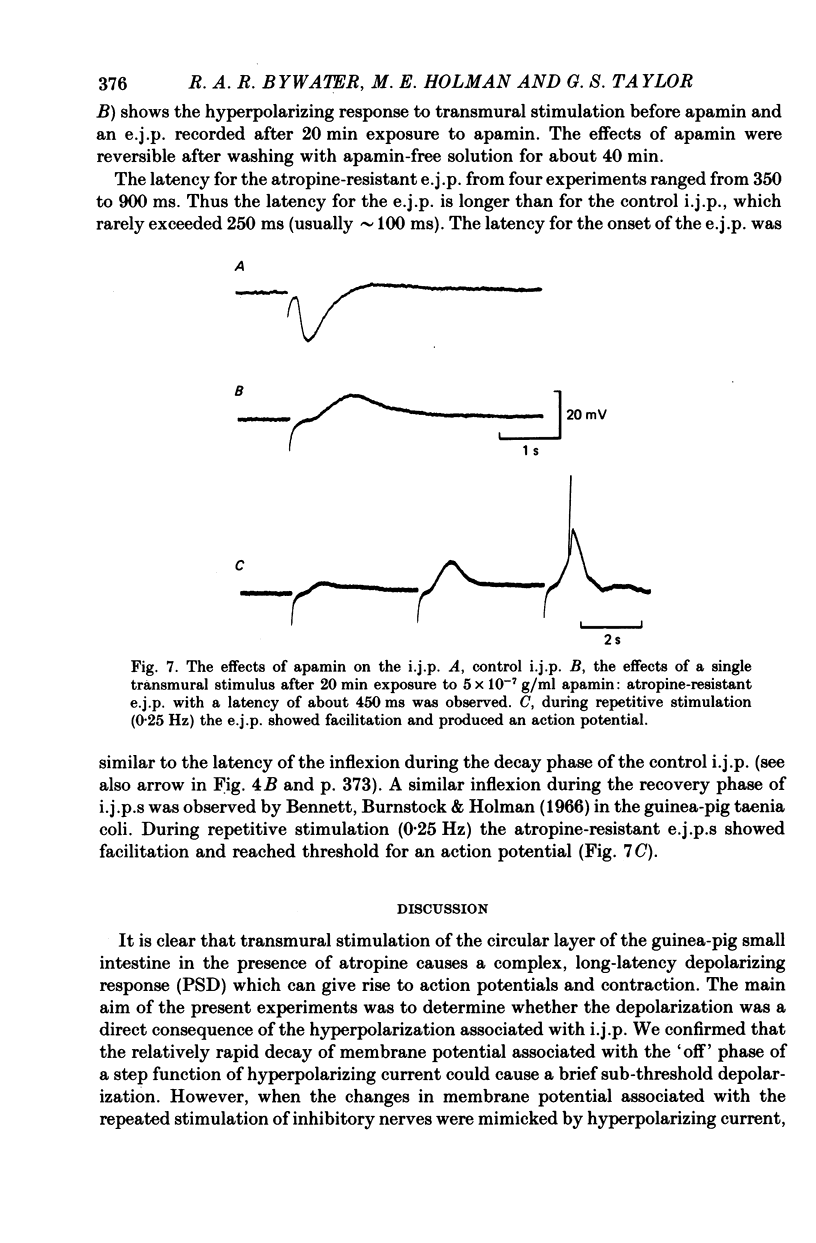
7. The neurotoxin apamin reversibly abolished the i.j.p. and unmasked a transient excitatory junction potential (e.j.p.) with a variable latency (350-900 ms).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Paddle B., Staszewska-Barczak J. Evidence that prostaglandin is responsible for the 'rebound contraction' following stimulation of non-adrenergic, non-cholinergic ('purinergic') inhibitory nerves. Eur J Pharmacol. 1975 Apr;31(2):360–362. doi: 10.1016/0014-2999(75)90060-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. The passive membrane properties and excitatory junction potentials of the guinea pig deferens. J Physiol. 1980 Mar;300:303–316. doi: 10.1113/jphysiol.1980.sp013163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A., van den Akker J. Effects of prostaglandin E2 and indomethacin on the rebound of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Apr 15;55(2):143–148. doi: 10.1016/0014-2999(79)90386-8. [DOI] [PubMed] [Google Scholar]
- Franco R., Costa M., Furness J. B. Evidence for the release of endogenous substance P from intestinal nerves. Naunyn Schmiedebergs Arch Pharmacol. 1979 Apr;306(3):195–201. doi: 10.1007/BF00507103. [DOI] [PubMed] [Google Scholar]
- Franco R., Costa M., Furness J. B. Evidence that axons containing substance P in the guinea-pig ileum are of intrinsic origin. Naunyn Schmiedebergs Arch Pharmacol. 1979 May;307(1):57–63. doi: 10.1007/BF00506552. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., McKirdy H. C. Two descending nerve pathways activated by distension of guinea-pig small intestine. J Physiol. 1975 Jan;244(1):113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Weinrich J. P. The effects of calcium and magnesium on inhibitory junctional transmission in smooth muscle of guinea pig small intestine. Pflugers Arch. 1975 Oct 28;360(2):109–119. doi: 10.1007/BF00580534. [DOI] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A. The effect of the phenyl phosphonate N-0164 on prostaglandin action and on post inhibitory excitation in the taenia of guinea-pig caecum. Eur J Pharmacol. 1980 Mar 21;62(2-3):157–166. doi: 10.1016/0014-2999(80)90272-1. [DOI] [PubMed] [Google Scholar]
- Shuba M. F., Vladimirova I. A. Effect of apamin on the electrical responses of smooth muscle to adenosine 5'-triphosphate and to non-adrenergic, non-cholinergic nerve stimulation. Neuroscience. 1980;5(5):853–859. doi: 10.1016/0306-4522(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kuriyama H. Electrical and mechanical properties of longitudinal and circular muscles of the guinea-pig ileum. Jpn J Physiol. 1975;25(6):759–773. doi: 10.2170/jjphysiol.25.759. [DOI] [PubMed] [Google Scholar]
- Tomita T. Conductance change during the inhibitory potential in the guinea-pig taenia coli. J Physiol. 1972 Sep;225(3):693–703. doi: 10.1113/jphysiol.1972.sp009964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirova I. A., Shuba M. F. Vliianie strikhnina, gidrastina i apamina na sinapticheskuiu peredachu v gladkomyshechnykh kletkakh. Neirofiziologiia. 1978;10(3):295–299. [PubMed] [Google Scholar]
- Wood J. D., Marsh D. R. Effects of atropine, tetrodotoxin and lidocaine on rebound excitation of guinea-pig small intestine. J Pharmacol Exp Ther. 1973 Mar;184(3):590–598. [PubMed] [Google Scholar]


