Table 1.
Torsion angle structural constraints in f-MLF-OH determined by 3D MAS dipolar-chemical shift experiments
| Residue | Angle | Data type | Most likely solutions, ° | Less likely solutions, ° |
|---|---|---|---|---|
| Met | φ | H-Ni–C –H –H |
−150 ± 2 | −6 ± 2 |
| −90 ± 2 | ||||
| ψ | N–C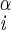 –C′i–N –C′i–N |
±157 ± 1 | NA | |
| 108 ± 18 | ||||
| −151 ± 10 | ||||
H–Ni+1–C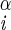 –H –H |
161 ± 4 | 78 ± 5 | ||
| −10 ± 8 | ||||
| 24 ± 11 | ||||
| χ1 | H–C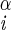 –C –C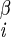 –H2 –H2
|
−163 ± 3 | 163 ± 3 | |
| −77 ± 3 | −43 ± 3 | |||
| χ2 | H2–C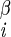 –C –C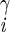 –H2 –H2
|
±169 ± 2 | NA | |
| Leu | φ | H–Ni–C –H –H |
−94 ± 2 | NA |
| −146 ± 2 | ||||
| ψ | N–C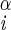 –C′i–N –C′i–N |
±91 ± 4 | ±45 ± 4 | |
| ±120 ± 4 | ±65 ± 5 | |||
H–Ni+1–C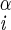 –H –H |
−69 ± 4 | −178 ± 7 | ||
| −51 ± 4 | −59 ± 10 | |||
| ±177 ± 3 | ||||
| χ1 | H–C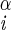 –C –C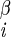 –H2 –H2
|
−57 ± 3 | NA | |
| −64 ± 3 | ||||
| ±173 ± 3 | ||||
| χ2 | H2–C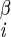 –C –C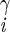 –H –H |
−65 ± 4 | NA | |
| −56 ± 4 | ||||
| Phe | φ | H–Ni–C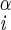 –H –H |
−163 ± 2 | −45 ± 6 |
| −77 ± 2 | 162 ± 2 | |||
| χ1 | H–C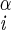 –C –C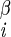 –H2 –H2
|
68 ± 4 | NA | |
| 52 ± 4 |
Four types of 3D experiments were performed, involving sets of nuclei A–B–C–D: 1H–15N–13C–1H (18), 1H–13C–13C–1H (41), 15N–13C–13C–15N (38, 39), and 1H–15N–15N–1H (23). In each experiment, B–C 2D chemical shift planes were recorded as a function of the dipolar mixing time between nuclei A–B and C–D. The modulation of the B–C cross–peak intensity reported on the relative orientation of the A–B and C–D dipole vectors, and therefore the A–B–C–D torsion angle (assuming invariant bond lengths and angles). Each experiment yielded several types of data, as listed in column three. Because of mirror plane symmetry, multiple solutions are possible in each experiment. Monte Carlo simulations (18) were performed, with a minimum of 10,000 iterations, to determine all possible solutions. Solutions were grouped within local minima; those that occurred in more than 20% of Monte Carlo simulations are listed as most likely solutions (with ±1 σ precision), whereas those that occurred less often are indicated as less likely solutions. [The results in cases where the B and C nuclei were not directly bonded (e.g., H–Ni–C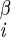 –H) depended on two intervening torsion angles (e.g., φ and χ1) in a coupled manner; several such 2D solution spaces were included in the final calculations, but are not shown here. The determinations of ψi by means of H–Ni+1–C
–H) depended on two intervening torsion angles (e.g., φ and χ1) in a coupled manner; several such 2D solution spaces were included in the final calculations, but are not shown here. The determinations of ψi by means of H–Ni+1–C –H data presumed a trans peptide bond (ω = 180°).] NA, not applicable.
–H data presumed a trans peptide bond (ω = 180°).] NA, not applicable.
