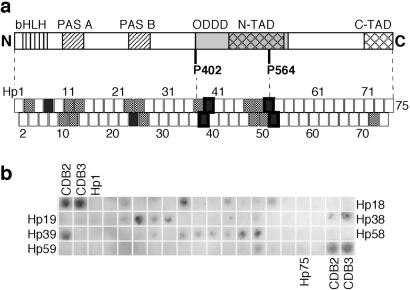
Fig 1.
Binding of p53c to an immobilized peptide array screening the HIF-1α sequence. (a) Map of how the array peptides correlated to the HIF-1α structure. The peptides have a sequence overlap with their neighbors and are numbered Hp1–75. HIF-1α consists of the following domains: DNA-binding domain basic helix–loop–helix (bHLH), dimerization domains PAS A and PAS B, oxygen-dependent degradation domain (ODDD), and two transactivation domains (N-TAD and C-TAD). P402 and P564 act as switches at oxygen-regulatory hydroxylation. HIF-1α-derived peptides with strong binding signals are indicated by filled boxes, and weakly binding peptides are indicated by dotted boxes. Peptide boxes with a thick frame have been analyzed in soluble form. (b) Average of three immunoblot experiments of p53c bound to HIF-1α peptide array. The 9-mer peptides CDB2 and CDB3 (15) were used as positive controls.