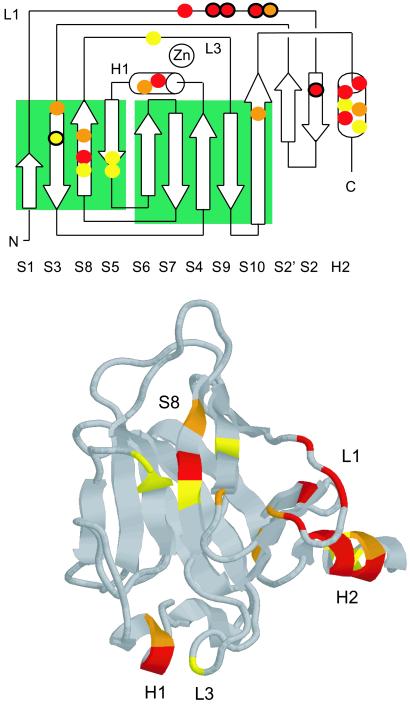
Fig 3.
Binding of HIF-1α-derived peptides to p53c analyzed by NMR. (Upper) Topology diagram (22) displaying residues in p53c with difference in shift after binding Hp39. Red, strong shifts (δ-1H > 0.034 ppm or δ-15N > 0.15 ppm) by H115, G117, T118, V122, Y126, H178, H233, G279, R280, and T284; orange, medium shifts (δ-1H > 0.022 ppm or δ-15N > 0.135 ppm) by T123, T140, H179, T231, V272, and R283; yellow, weak shifts (δ-1H > 0.014 ppm or δ-15N > 0.075 ppm) by V143, E198, G199, Y234, G244, R282, and E285; black circles, Hp39 and Hp39-1 showed identical binding, whereas Hp39-2 was significantly weaker, G117, T118, V122, T123, Y126, and V143. (Lower) Picture of p53c with residues colored as described for Upper.