Fig 3.
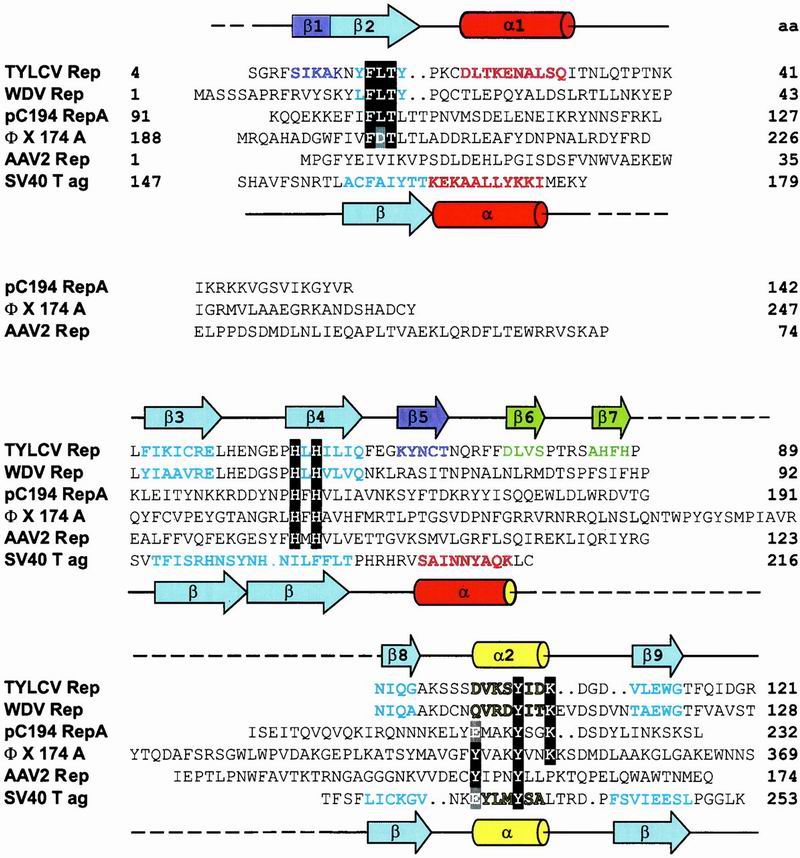
Structure-based sequence alignment of the catalytic domains of Rep proteins from TYLCV, WDV, pC194, ΦX, AAV2, and the DBD of SV40 T-ag. Amino acids of the motifs I, II, and III (28) are highlighted in black. The catalytic tyrosine(s) and equivalent residues in SV40 T-ag are highlighted in gray. Secondary structure elements present in the TYLCV Rep domain and SV40 T-ag 3D structures are indicated by cylinders (α-helices) and arrows (β-strands) above and below the sequence alignment, respectively. Amino acids in β-strands and α-helices of TYLCV Rep and SV40 T-ag, as well as predicted ones, are colored according to their location in the structure (see Fig. 2 B and C). Residue numbers are given at the end of each line. (GenBank accession nos: TYLCV Rep, CAA43466; WDV RepA, CAA57625; PCV2 Rep, AAC59462; pC194 RepA, NP_040435; IS91 TnpA, CAA34970; ΦX174 A, NP_040703; AAV2 Rep68, AAC03774; SV40 T-ag, P03070).
