Abstract
The senescence of immune cells, including macrophages, that accompany the initiation and development of tumors has become a novel research hotspot. Recently, studies have reported the molecular characteristics of senescent macrophages (sMACs) in the tumor microenvironment (TME), including cell cycle arrest, senescence-associated secretory phenotype (SASP), and senescence-associated β-galactosidase phenotype (SA-β-gal), and these characteristics not only suggest that sMACs are functionally rich in the TME, but also have the potential to become biomarkers for the identification of sMACs. The in-depth study and analysis of sMACs dialogue and mediating the changes of signaling pathways related to tumor and immune cells will help us to better understand the balance between tumor and aging. Here, we review recent advances in sMACs, including phenotypical molecular characteristics, potential functions and intervention approaches.
Subject terms: Senescence, Cancer microenvironment
Facts
Senescent macrophages are a component of the tumor immune microenvironment and play a multifaceted role.
Senescent macrophages may exhibit molecular differences in various tumors, and their identification requires the use of single-cell omics or combinatorial biomarker strategies.
Senescent macrophages regulate the infiltration and function of immune cells in tumors by secreting factors such as SASP or by expressing proteins like PDL1.
Targeting senescent macrophages is a novel and promising therapeutic strategy in oncology.
Open questions
How to establish a reliable methodological framework to achieve accurate qualitative and quantitative assessment of senescent macrophages?
What are the detailed molecular mechanisms of senescent macrophages in promoting tumor initiation, progression, and immunomodulation?
How to develop new therapeutic strategies based on senescent macrophage-specific targets to achieve effective antitumor responses?
Introduction
Senescence has been recognized as one of the novel hallmarks of cancer. Cellular senescence occurs when proliferating cells enter a stable state of cell cycle arrest under conditions of damage or stress. This state encompasses a range of physiological processes and is intricately linked to tumors, Alzheimer’s disease, and other age-related diseases (ARDs) [1–4]. The deficiency of endogenous telomerase activity and telomere attrition are fundamental factors that contribute to cellular dysfunction and senescence. Hallmarks of senescence include irreversible cell cycle arrest, buildup of DNA damage, enhanced lysosomal activity, and metabolic dysregulation linked to mitochondrial impairment, including alterations in silent information regulator and mechanistic target of rapamycin (mTOR) signaling pathways [2, 3, 5, 6]. Abnormal cytokines or chemokines are secreted by senescent cells, collectively termed the senescence-associated secretory phenotype (SASP), which plays a critical role in immune-cell communication [3, 7–9]. SASP refers to the pro-inflammatory molecules secreted by senescent cells. While some investigations proposed that cellular senescence facilitates angiogenesis, tissue healing, and suppression of tumor growth [10–12], accumulating evidence reveals that, during chronic injury, senescent cells can evade immune surveillance by secreting SASP. This evasion fosters the accumulation of senescent cells, which disrupts the tissue microenvironment and may contribute to the onset of various ARDs, including malignancies [1, 7, 10, 13].
The tumor microenvironment (TME) refers to the local region in which tumor cells reside, incorporating a diverse array of non-tumor cell types, extracellular matrix (ECM) components, vascular networks, and soluble factors. This intricate milieu is critical in modulating tumor behavior and influencing therapeutic responses [14, 15]. TME-associated senescent cells manifest a dualistic role: on the one hand, senescence TME statue can inhibit tumor progression by slowing their proliferation [11]; conversely, the dynamic interplay of immune cell functions and cytokines produced by senescent cells allows these senescence-associated factors to modify the immune escape mechanisms within the TME, thereby significantly promoting tumor growth and the spread of cancer to distant sites [7, 10, 16, 17].
Macrophages are essential components of innate and adaptive immunity and are one of the major infiltrating immune cells in TME. These macrophages are known as tumor-associated macrophages (TAMs) and play a dual role in tumor initiation and progression, acting as promoters and tumorigenesis suppressors [18, 19]. Macrophages can be classified into two distinct subtypes, also known as the polarization states of macrophages: M1 type and M2 type (Fig. 1). M1 macrophages are classically triggered, pro-inflammatory cells that exert direct tumor-suppressive effects by secreting pro-inflammatory cytokines, such as interleukin-6 (IL-6), and generating reactive oxygen species and reactive nitrogen species, all of which contribute to the amplification of anti-tumor immune responses. In contrast, M2 macrophages release immunosuppressive mediators, including IL-4, IL-10, and transforming growth factor-beta (TGF-β), which inhibit T cell and natural killer (NK) cell functionality, promote angiogenesis, and facilitate tumor cell invasion, thereby contributing to tumor progression [19–21].
Fig. 1. Inducing factors and related molecular characteristics of different macrophage phenotypes.
M0 macrophages are derived from monocytes under the stimulation of PMA. M0 macrophages can differentiate into M1 macrophages upon induction by IFN-γ and LPS or M2 macrophages under the influence of IL-4 and IL-10, accompanied by corresponding molecular characteristic changes. Under stimulation by factors such as UV radiation, stress, or specific chemical agents, macrophages can transition into senescent macrophages, exhibiting general senescence-associated phenotypic changes such as increased expression of P16 and P53, elevated secretion of SASP, increased β-galactosidase activity, enhanced DNA damage in the nucleus, and reduced Lamin B expression. Additionally, they display specific markers of senescent macrophages, including elevated expression of CD22, CD38, PD-L1, CXCR1, RACK-1, LYVE-1, and AKR1B1, as well as decreased expression of MHC II and TLRs. The polarization process from M0 macrophages to senescent macrophages is characterized by a gradual decline in viability and a progressive increase in the degree of senescence.
Contrary to the disordered proliferation of tumor cells, immune cells such as macrophages present a state of exhaustion or senescence-related reprogramming in TME. In 1978, Johnson et al. noted a significant reduction in the cytoplasmic spreading ability of macrophages in aged mice [22]. Subsequently, several studies have established that, as aging progresses, macrophages exhibit a progressive decline in phagocytic capacity, respiratory burst activity, levels of toll-like receptors (TLRs) and MHC class II (MHC-II), responsiveness to antigenic triggers, and the release of pro-inflammatory chemokines and cytokines [23–28], suggesting that the senescence of macrophages occurs with aging, and the molecular changes of senescent macrophages (sMACs) disrupt the regular immune cell dialog. In addition, in vivo animal experiments showed that elimination of sMACs could inhibit tumor growth, suggesting the clinical significance of combination therapy targeting sMACs [29, 30]. Nonetheless, there is still a lack of consensus regarding the characteristic phenotypes of sMACs within the TME and their mechanistic roles. This review aims to provide an overview of the current understanding of the tumor infiltration-sMACs, summarize the molecular characteristics and functional abnormalities of sMACs, and discuss the potential roles and interventions of sMACs.
Molecular characteristics of senescent macrophages (sMACs)
Senescent cells may occur at any stage of life, and real-time quantitative detection of cellular senescence is an important prerequisite for research. In 2019, the International Cell Senescence Association established an extensive definition of cellular senescence, outlined its hallmark characteristics, and recommended detection approaches, emphasizing the necessity for a multifaceted assessment that integrates various senescence markers to identify senescent cells accurately [3]. But, sMACs is still not well defined and guideline biomarkers are lacking. Evidence suggests that, like most senescent cells, sMACs secrete mediators such as IL-6 and TNF that enhance a proinflammatory phenotype. Additionally, sMACs show increased expression of inflammatory cytokine genes following TLRs activation, likely resulting from the reduced ability of sMACs to downregulate TLRs signaling pathways [31–34]. Hall et al. demonstrated a marked elevation in p16 and β-galactosidase levels in senescent M2 macrophages, supporting earlier hypotheses regarding the potential phenotypic resemblance linking senescent cells to M2 macrophages [35]. Crystal et al. found that chemotherapy-induced senescent tumor cells acquire a survival advantage by engulfing neighboring cells, exhibiting gene expression changes associated with phagocytic function [36]. Additional research has indicated that macrophages may undergo cell cycle arrest simultaneously with acquiring a pro-inflammatory phenotype, leading to the term “senescence-like macrophages” [37, 38]. The above observations highlight an aging polarized state or senescence of macrophages distinct from M1/M2 polarization. Collectively, the differentiation factors and specific molecules of sMACs are significantly different from those of M1-type and M2-type macrophages (Fig. 1).
Cell cycle-related genes
Cell cycle arrest, mainly governed by the p16Ink4a/RB and p53/p21CIP1 signaling pathways, represents a fundamental characteristic of senescent cells [39, 40]. A strong correlation has been demonstrated between p16 expression at both the RNA and protein levels and the state of cellular senescence [4]. Cellular senescence is regarded as a permanent state of arrest that cannot be reversed by known physiological stimuli [41]. Grosse et al. developed a knock-in mouse model, which specifically targets p16Ink4a and identified a population of macrophages in these mice with significantly increased of p16Ink4a expression [38]. Liu et al. further observed that, as aging progresses and inflammation accumulates, the subpopulation of macrophages with elevated p16Ink4a levels gradually increased, accompanied by the upregulation of molecules indicative of senescence [42]. Moreover, in vitro experiments utilizing oxidized low-density lipoprotein (Ox-LDL) to induce senescence in RAW264.7 mouse macrophage cell lines demonstrated significant upregulation of p53, p21, and p16 expression levels, suggesting that macrophage senescence also presents senescence-associated phenotypic characteristics [43]. Hall et al. found that tissue macrophages can express high p16Ink4a expression when subjected to alginate encapsulation, with the associated increases in senescence-associated β-galactosidase (SA-β-gal) and p16Ink4a levels under these conditions being reversible. These macrophages remain responsive to polarization stimuli, suggesting that high expression of p16Ink4a in macrophages does not necessarily indicate senescence but instead represent a physiological adaption to immune activation [35]. Milan R. et al. observed that mouse microglia exhibit shortened granules and markers of DNA damage after multiple passages, but such features were not detected in microglia from aged mice. Additionally, the low p16 expression found in both early-passage microglia and those isolated from 3-month-old mice further indicate p16 may not serve as a definitive marker for macrophage senescence [44]. PMA-induced macrophages derived from THP-1 cells in vitro exhibit cell cycle arrest in the G2 or M phase [45]. Human macrophages stimulated by the TLR4 agonist, lipopolysaccharide (LPS), exhibit a secretory pro-inflammatory phenotype accompanied by cell cycle arrest [46]. These results suggest cell cycle arrest may be a primary characteristic of terminally differentiated macrophages [37]. While it remains unclear whether cell cycle arrest definitively signifies sMACs, current recommendations suggest evaluating macrophage senescence status by combining the analysis of p16 or p21 alongside additional relevant senescence markers.
Lysosomal-senescence-associated β-galactosidase axis
One of the typical features of senescent cells, including sMACs, is the expansion of the lysosomal compartment, leading to significantly enhanced β-galactosidase activity [47–49]. Although this marker can also be expressed in other cellular physiological processes, SA-β-gal has become one of the most commonly used methods for detecting cellular senescence due to its simplicity and relatively high specificity [3, 6]. However, an increasing body of evidence suggests that normal macrophages may also demonstrate enhanced lysosomal capacity. Kopp et al. reported the detection of SA-β-galactosidase-positive expression in osteoclasts across various age stages in mice, which may result in misleading background interpretations [50]. This phenomenon has similarly been observed in the liver’s brain microglia and Kupffer cells, indicating that lysosomal expansion represents a key characteristic of macrophages [51, 52]. Likewise, the differentiation of monocytes into macrophages is predominantly marked by an increase in the quantity of lysosomes [45]. Moreover, several studies have shown that macrophages exhibit a marked increase in SA-β-gal expression under stimulatory conditions, such as radiation, accompanied by additional senescent phenotypes [53, 54]. There was a significant negative correlation between the expression of KI67 and SA-β-gal in expanded culture macrophages in vitro [55]. Additionally, another report noted that in the tissues of older mice, most cells co-expressing SA-β-gal and p16 were macrophages [56], suggesting the potential relevance of SA-β-gal for studies on sMACs.
Currently, a standardized protocol for staining β-galactosidase in sMACs is lacking. Moreover, a key lysosomal characteristic linked to cellular senescence is the accumulation of lipofuscin, which can be effectively detected using Sudan Black B (SBB) staining and utilized in paraffin-embedded tissue sections. Compared to SA-β-galactosidase staining, SBB staining is more widely applicable but demonstrates lower specificity [57]. In addition. Factors such as temperature and fixative solution during specimen processing may lead to the inactivation of SA-β-gal, which is the difficulty for its stable detection.
Senescence-associated secretory phenotype
The collection of cytokines (IL-1a, IL-6), chemokines (CXCL12), growth factors (FGF, VEGF), matrix metalloproteinases (MMPs), and regulatory factors like TGF-β are collectively known as SASP [2, 58–60]. Studies demonstrated that both sMACs and other senescent cells can secrete SASP, and its plays a crucial role in sustaining chronic, systemic, low-grade inflammation and exacerbating the dysregulation of inflammatory responses during aging. This contributes to the pathogenesis and progression of various ARDs, including tumor [61]. Serval studies have elucidated the significant roles of senescent cells in the initiation and progression of tumors [62]. The earliest evidence demonstrating the paracrine tumorigenic activity of senescent cells was observed in senescent fibroblasts [63]. Senescent stromal cells are known to release elevated amounts of MMP-1, MMP-3, and MMP-10, which support tumorigenesis by remodeling the ECM [4]. In addition, senescent cells secrete the SASP, notably IL-6, which upregulates HLA-E expression, thereby suppressing the immune activity of NK cells and T cells against senescent cells [64]. By releasing IL-6 and IL-8, the SASP promotes the development of precancerous chronic inflammation, facilitating epithelial-mesenchymal transition (EMT) [65]. Furthermore, the SASP can recruit myeloid-derived suppressor cells (MDSCs) by releasing various cytokines, thereby exacerbating the inhibition of anti-tumor immune responses [66].
Recently, by performing single-cell sequencing of thymoma tissues, we identified a macrophage subset expressing CCL3+, indicating the possible presence of sMACs that secrete the SASP within the TME [67]. Research suggests that LPS-induced sMACs exhibit significantly elevated levels of pro-inflammatory factors, such as TNF-α, IL-6, COX-2, and PGE2 [46, 68]. Scott et al. employed a novel P16-FDR mouse model, revealing that macrophages exhibiting senescent features express a distinct array of pro-tumor SASP factors, including BMP2, IL-10, CCL2, CCL7, CCL8, and CXCL13, which facilitate lung cancer progression by activating the KRAS signaling pathway [69]. Notably, SASP-associated CCL7 and IL-10 have been demonstrated to significantly promote tumor cell invasion and metastasis [70–72]. Liu et al. discovered a population of macrophages in the aging liver that expresses CXCL2 and possess the capacity to secrete SASP factors; these sMACs utilize the CXCL2-CXCR2 axis to attract neutrophils, which in turn further stimulate the formation of neutrophil extracellular traps (NETs) by secreting IL-1β and TNF-α; the pathological accumulation of NETs is associated with liver damage, which may act as an early event in the development of hepatocellular carcinoma [73–75].
Discovery and identification strategies of sMACs
Discovery of sMACs by single-cell multi-omics
The rapid development of single-cell multi-omics technology in recent years has enriched our understanding of the identification of cell subpopulations in tissues and their evolutionary relationships [76]. By constructing a single-cell atlas of macrophages and performing unsupervised clustering analysis, sMACs with aging characteristics can be highlighted from the single-cell atlas [77].
Brittany et al. performed single-cell transcriptomic analysis of healthy breast tissue and found significant age-related expansion of myeloid cell populations, particularly M2-like macrophages. These cells progressively accumulated during aging and exhibited high expression of chemokines CCL7 and CCL8 at the transcriptomic level [78]. As CCL8 is a canonical component of the SASP, this subpopulation likely possesses senescent features and may represent a functionally distinct population of sMACs involved in shaping the aging tissue microenvironment [79]. Matthew et al. used single-cell and epigenetic analysis of an aged mouse lung cancer model and found that the age-related decline in DNMT3A expression in aged myeloid cells activates IL-1α expression, thereby promoting tumorigenesis, suggesting a potential mechanism of aging-related myeloid cell population in carcinogenesi [80].
Additionally, recent advances in technologies such as single-cell live imaging combined with mass spectrometry (SCLIMS) enable simultaneous acquisition of cellular metabolic profiles and phenotypic states, offering an innovative tool for studying metabolic reprogramming during cellular senescence, with promising applications in sMACs research [81].
Combining biomarkers to identify sMACs
The omics screening technology improves the discovery probability of sMACs, but the qualitative and quantitative examination of the distribution observation in the tissue is key to carrying out in-depth research. Considering the cell species and senescence characteristics of sMACs, such as macrophage markers (e.g., CD14 and CD68) and senescence signatures (e.g., P16, SASP factors, and lysosomal markers), a combination of at least two of the above biomarkers is recommended for the identification of sMACs. Therefore, the sMACs marked with multiple fluorescence can be detected by multiple immunofluorescence or flow cytometry, and the quantitative data of sMACs can be obtained by automatic calculation of software or algorithms. In addition, M1-type or M2-type macrophage markers could also be designed in the experiment for co-staining to increase the reliability of obtaining the sMAC ratio from the perspective of negative fluorescence.
Phenotypic and functional abnormalities of sMACs
As various physiological functions decline with human aging, sMACs also manifests as a decline in multiple functions, including phagocytosis, oxidative stress resistance, cytokine secretion, tissue repair, autophagy, respiratory capacity, and the expression of TLRs and MHC-II molecules [27, 82].
Macrophages, essential components of the innate immune cells, are highly efficient at engulfing and eliminating pathogens, playing a crucial role in the immune defense. Research shows that when cultured for extended periods, sMACs significantly decrease their phagocytic capacity and produce fewer inflammatory cytokines when stimulated with LPS [55]. Yohko et al. demonstrated that the impaired activity of the aging-associated gene P53 plays a pivotal role in the reduced phagocytic capacity of macrophages in aged mice. This highlights that aging directly affects the ability of macrophages to perform phagocytosis [83]. Several animal studies further validate this idea, revealing that macrophages in aged mice have a diminished capacity for phagocytosis, leading to increased vulnerability to diseases like influenza [84]. Pluvinage et al., through CRISPR-Cas9 screening and RNA-seq analysis, identified a marked age-related increase in CD22 expression in microglia, and inhibition of CD22 was shown to improve the clearance of myelin debris, amyloid-β, and α-synuclein fibers. These findings implicate CD22 as a negative regulator of microglial phagocytic activity in the central nervous system, highlighting its potential involvement in the pathogenesis of age-related neurodegenerative disorders [85]. The work of Aires et al. also suggests that the expression of CD22 in aging microglia may potentially impact the occurrence of neurodegenerative lesions. The authors’ findings indicate that CD22 expression may serve as a significant marker linked to macrophages’ phagocytic function and aging.
sMACs is also tied to a decrease in the expression of TLRs and MHC-II. TLRs can recognize conserved elements in pathogens, activating immune cells, such as macrophages and dendritic cells [25]. The levels of TLRs expressed in splenic and peritoneal macrophages from aged mice has been shown to be significantly reduced, and following stimulation with TLR ligands, the production of pro-inflammatory factors, such as IL-6 and TNF-α is markedly diminished [26]. Additionally, the interaction between TLRs and adenosine A receptors plays a pivotal role in regulating macrophages’ secretion of vascular endothelial growth factor (VEGF) and other angiogenic factors. Consequently, the diminished expression of TLRs in macrophages may impair their capacity for effective wound healing [86–88]. Moreover, macrophages present antigenic peptides via MHC-II molecules to helper T cells, initiating adaptive immune responses. Numerous studies have indicated that aging reduces macrophages’ ability to express MHC-II genes in response to IFN-γ stimulation [89–91].
The respiratory capacity of macrophages also declines with age. Enhanced p38 mitogen-activated protein kinase (MAPK) activity in sMACs inhibits T-cell immunoglobulin mucin protein 4 (TIM4) receptor’s expression, impairing its ability to phagocytose apoptotic bodies. This attenuation in clearance mechanisms can hinder the resolution of acute inflammation, potentially triggering the pathogenesis of chronic inflammatory diseases, including Alzheimer’s disease and atherosclerosis [92]. Moreover, sMACs exhibits activation of the kynurenine pathway (KP), which suppresses quinolinic acid phosphoribosyl transferase (QPRT) activity and impairs nicotinamide adenine dinucleotide (NAD) synthesis. This leads to a reduction in mitochondrial respiration capacity and fosters a pro-inflammatory phenotype [93–96], further exacerbating the depletion of tissue NAD levels through increased activity of the NAD-consuming enzyme CD38 [97]. The above metabolic indicators, the TIM4 receptor, and CD38 may also serve as biomarkers for sMACs.
Potential molecular regulatory mechanisms of sMACs in shaping the TME
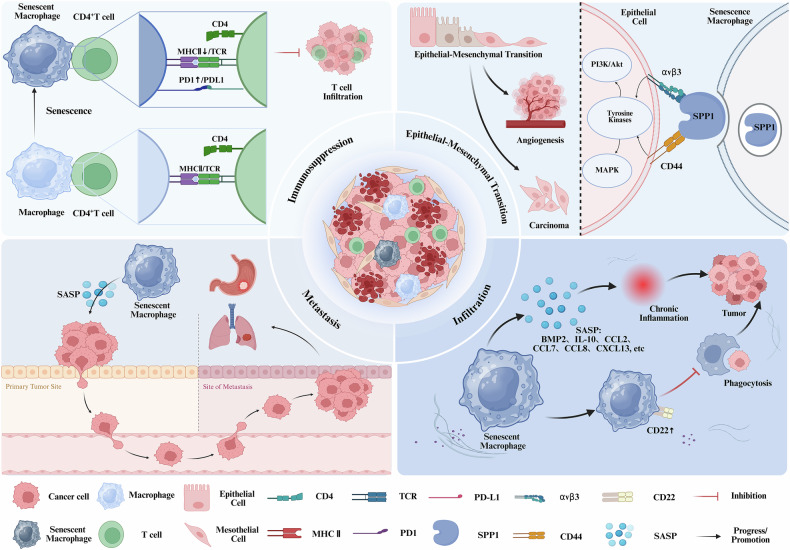
The disordered proliferation of malignant cells is accompanied by the senescence of other cells, suggesting that inhibition and restoration may be equally important in treating tumors. Lelinh et al. conducted experiments demonstrating that mesothelioma exhibited a more rapid proliferation rate in aged mice, accompanied by a higher density of infiltrating TAMs within the tumors [98]. Daniella et al. analyzed data from The Cancer Genome Atlas for prostate adenocarcinoma (TCGA-PRAD) and found that the expression levels of macrophage-related genes, including CD163 and VSIG4, were linked to tumor recurrence, and were significantly higher in prostate cancer tissues from older patients [99]. Additionally, Li et al. presented that in tumor tissues of older patients, the infiltration rate of CD68+ CD206+ cells was higher than that in younger patients, and sMACs co-cultured with tumor cells could enhance the size of tumors [100]. An increasing body of evidence indicates that sMACs are prevalent in tumors, where they support a pro-tumor microenvironment phenotype by activating multiple molecular pathways (Fig. 2).
Fig. 2. The potential role of senescent macrophages on tumors.
(1) Senescent macrophages suppress T cell proliferation through the downregulation of MHC II expression and the upregulation of PD-1 expression. (2) Senescent macrophages promote epithelial-mesenchymal transition (EMT) by increasing SPP1 expression, thereby facilitating tumor growth and angiogenesis. (3) Senescent macrophages’ secretion of senescence-associated secretory phenotype (SASP) factors drives tumor metastasis. (4) Senescent macrophages promote tumorigenesis and progression by upregulating CD22 expression, reducing phagocytic capacity, and inducing chronic inflammation through SASP secretion.
Programmed death-ligand 1 (PD-L1)
Julia et al. conducted a single-cell analysis of murine lung tissues and found a significant increase in alveolar macrophages in aged mice. In a model of chronic pneumonia, the stability of programmed death-ligand 1 (PD-L1) protein was increased in sMACs in lung epithelial cells; the protein degradation was slowed via P16-mediated inhibition of CDK4/6 pathways [101]. In the TME, both tumor cells and non-tumor cells express PD-L1. While this mechanism maintains immune system equilibrium in healthy physiological states, it facilitates immune escape by tumor cells in the TME, consequently supporting their proliferation and metastasis [102–105]. In recent years, blocking the PD-L1/PD-1 pathway has shown significant anti-tumor effects in advanced cancer patients; this pathway has become a direction of intense development of new immune checkpoint blockade therapies and their combinations. Several antibodies targeting PD-1/PD-L1 are undergoing clinical trials, representing a crucial component in targeted therapy [106, 107]. However, many patients are resistant to this treatment, particularly those with solid tumors, which may be primarily related to irreversible T cell exhaustion, dysfunctional MHC expression and activity, along with resistance to IFN-γ signaling [108]. Since macrophages represent the predominant population of innate immune cells within the TME, and are closely linked to tumorigenesis and chronic inflammation, targeting PD-L1 expression in sMACs may provide innovative strategies with promising clinical outcomes in tumor immunotherapy.
Secreted phosphoprotein 1 (SPP1)
Single-cell RNA sequencing of aged murine skeletal muscle by Bi et al. identified an SPP1high macrophage subset enriched in senescent tissue. This population showed elevated expression of senescence markers and activation of VEGF and lipid metabolism pathways, indicating a potential role in immunometabolic remodeling and tissue homeostasis during aging [109]. Yu et al. constructed an aging-related prognostic model for colorectal cancer (CRC) based on TCGA and GEO databases. The authors found that patients in the high-risk group had an abundance of macrophages in their tumor tissues, exhibiting immune suppression characteristics. Data analysis further identified a distinct subpopulation of macrophages with elevated SPP1 expression in high-grade tumors, the macrophages concurrently displayed pronounced features of the SASP. These macrophages were surrounded by senescent tumor cells, indicating a potential correlation with poor prognosis linked to cellular senescence. SPP1, or osteopontin, is a multifunctional secreted phosphoglycoprotein primarily expressed in immune cells like macrophages and dendritic cells, though it can also be highly expressed in certain tumor cells. Extensive research has demonstrated that the expression levels of SPP1 in TAMs are positively correlated with tumorigenesis, invasion, metastasis progression, and the exacerbation of adverse clinical prognoses. Furthermore, the metabolic characteristics of SPP1+ macrophages and the functional properties of the ECM exhibit marked alterations [110–112]. SPP1 induces the activation of the PI3K/Akt and MAPK pathways through its interaction with integrin αvβ3 and CD44, thereby facilitating EMT, and epigenetic modifications and contributing to the development of drug resistance. Therefore, SPP1 is considered a potential novel biomarker for TAMs and may even become an important therapeutic target [113–115]. Despite the extensive research on the critical role of SPP1 in TAMs, its relationship with sMACs requires additional experimental exploration.
C-X-C chemokine receptor 1 (CXCR1)
Luis et al. established a kirsten ratsarcoma viral oncogene homolog (KRAS) mutation-driven lung adenocarcinoma mouse model to identify a population of p16+CXCR1+aging macrophages within tumor tissue through single-cell sequencing analysis. This study identified p16⁺CXCR1⁺ sMACs as key mediators of immune suppression by secreting IL-8 and upregulating PD-L1, thereby promoting a tumor immune-cold microenvironment through T cell inhibition. Notably, the depletion of this senescence macrophage population significantly slowed the progression of lung adenocarcinoma, indicating that these p16+CXCR1+ macrophages are crucial in altering the TME and thus contributing to tumorigenesis. This study represents the first to propose CXCR1 as a potential biomarker for macrophage senescence in the context of lung adenocarcinoma [116]. CXCR1 is a seven-transmembrane G protein-coupled receptor (GPCR) that belongs to the GPCR superfamily. It primarily transmits inflammatory signals and regulates immune responses by recruiting and activating leukocytes [117]. A growing body of research has demonstrated that CXCL8 and its homologous receptors, CXCR1 and C-X-C chemokine receptor 2 (CXCR2), play significant roles in the occurrence and progression of various cancers, including breast cancer, prostate cancer, lung cancer, CRC, and melanoma. These chemokines and their receptors regulate the proliferation, invasion, and migration capacity of tumor cells via autocrine or paracrine mechanisms [118, 119]. In prostate cancer, the CXCL8/CXCR1 axis primarily drives tumor cell proliferation and demonstrates a significant association with unfavorable clinical outcomes [120]. Additionally, CXCR1 has been shown to be constitutively expressed in all cases of melanoma [121]. Thus, targeting CXCR1 may represent a novel strategy for treating various malignancies [122].
Receptor for activated C kinase 1 (RACK1)
RACK1, a key member of the RACK family, functions as a multifunctional scaffold protein that orchestrates diverse signaling pathways involved in cell cycle regulation, proliferation, migration, and senescence [123]. Studies have shown that RACK1 promotes the ubiquitination and degradation of p53 by recruiting MDM2 through its interaction with FGFR, thereby suppressing p53-dependent senescence [124]. It can also inhibit p21Cip1 expression by binding to Smad3, blocking p21-induced growth arrest [125]. These mechanisms, validated in lung cancer cells and neural stem cells, may similarly regulate macrophage proliferation and senescence.
In sMACs, RACK1 expression is closely associated with immune function. With aging, RACK1 levels decline in alveolar macrophages, impairing cytokine secretion such as tumor necrosis factor alpha upon LPS stimulation, and reducing the release of SASP components like CCL2, CCL5, and IL-6 [126]. This downregulation may contribute to immunosenescence and the shift of macrophages toward a tumor-promoting phenotype.
Moreover, decreased RACK1 expression has been associated with tumor progression across multiple cancers. For instance, Helicobacter pylori infection suppresses RACK1 via the integrin β1/NF-κB pathway in gastric cancer, facilitating inflammatory signaling and tumorigenesis [127]. In colorectal and pancreatic carcinoma, low RACK1 levels correlate with increased invasiveness and chemoresistance [128]. Together, these findings underscore RACK1’s dual role in maintaining immune homeostasis and suppressing tumorigenesis, suggesting its potential as a therapeutic target in sMACs-driven pathologies.
Lymphatic endothelial hyaluronan receptor 1 (LYVE1)
Linda et al. conducted single-cell RNA sequencing (scRNA-seq) analysis of skeletal muscle and found that the number of LYVE1 macrophages was greater than that of LYVE1+ macrophages in aged skeletal muscle. Moreover, the expression levels of pro-inflammatory markers (such as S100a8 and S100a9 mRNA) and senescence-associated markers (such as GPNMB and SPP1 mRNA) exhibited a significant positive correlation with the increased LYVE1- macrophages. This suggests that LYVE1 may be a key marker for distinguishing sMACs [129]. LYVE1 is a CD44 homolog encoded by the LYVE1 gene [130, 131]. In certain cancers, LYVE1 expression levels are significantly elevated, and accumulating evidence implicates it in the initiation and metastatic progression of the primary tumor. In particular, LYVE1 secreted by macrophages has been shown to inhibit melanoma cell proliferation and foster lymphangiogenesis, thereby providing further evidence for the potential role of LYVE1 aging macrophages in the pathogenesis and progression of malignancies [132, 133].
Aldo-Keto reductase family 1 member B1 (AKR1B1)
Zhang et al. analyzed TCGA gastric cancer data and identified five genes related to aging within the gastric cancer TME, among which AKR1B1 was notably included [134]. Subsequently, Jiang et al. used the CELLAGE and TCGA databases to analyze scRNA-seq markers, identifying 23 common aging-related macrophage genes in bladder cancer patients. The study further confirmed a significant association between the upregulation of AKR1B1 in sMACs and the occurrence and progression of bladder cancer [135]. In vitro experiments showed that hydrogen peroxide-induced sMACs significantly upregulated AKR1B1 expression, enhancing the proliferation and migration of gastric and bladder cancer cells [136]. Aldose-ketose reductase family 1 (AKR1) is a family within the aldose-ketose reductase (AKR) superfamily, and AKR1B1 is one of its subfamilies [137]. The aging-related tumor suppressor gene P53 has been implicated in modulating the expression of AKR1B1, thereby exerting influence over the metastatic characteristics of breast cancer [138]. Multiple studies have confirmed that AKR1B1 is overexpressed in various tumors. For instance, in lung cancer, suppression of AKR1B1 expression has been shown to restore tumor sensitivity to EGFR tyrosine kinase inhibitors (TKIs) [139]. Additionally, in endometrial and ovarian cancers, AKR1B1 expression levels are significantly correlated with cellular oxidative stress and prognosis. In macrophages, AKR1B1 upregulation supports ROS-NF-κB signaling, promoting SASP factor secretion and potentially enhancing the pro-tumorigenic effects of sMACs [140]. Nonetheless, the precise association between AKR1B1 and aging macrophages necessitates further investigation and empirical validation to elucidate the underlying mechanisms.
Novel strategies for cancer therapy targeting sMACs
Senolytics
The increasing evidence highlighting the close involvement of senescent cells in cancer pathogenesis necessitates exploring effective strategies for their targeted elimination (Fig. 3). Although senescent cells secrete pro-apoptotic SASP factors, these cells have been shown to resist apoptosis. It is hypothesized that senescent cells may evade clearance through protective anti-apoptotic pathways [141–143]. Bioinformatics analyses of proteomic and transcriptomic data from senescent and non-senescent cells have identified several senescent cell anti-apoptotic pathways (SCAPs), which mean survival pathways upregulated in senescent cells that help protect them from apoptosis, including the PI3Kδ/AKT, BCL-2/BCL-xL/BCL-W, and the p53/FOXO4a/p21/serpin [plasminogen activator inhibitor 1 and 2 (PAI-1, PAI-2)] pathways [142, 144]. Following the integration of SCAPs, and screening of drug libraries, numerous senolytic drugs have been identified, many of which are anticancer agents. These include tyrosine kinase inhibitors, such as dasatinib and quercetin (D + Q), and BCL-2 family inhibitors like venetoclax. These compounds have demonstrated promising effects in various studies [145–148].
Fig. 3. Senolytics and senomorphics eliminate senescent cells or suppress the SASP via multiple mechanisms.
D + Q, a tyrosine kinase inhibitor, induces apoptosis in senescent cells; Navitoclax and Fisetin promote apoptosis primarily through inhibition of Bcl-2 family proteins; FOXO4-DRI binds to FOXO4, disrupting its interaction with p53 to induce cell death; Niacin facilitates clearance by enhancing phagocytic activity; Rapamycin, Aspirin, and Metformin suppress SASP release by inhibiting NF-κB-related signaling pathways.
Currently, therapeutic strategies targeting sMACs are limited and still in the early stages of development. Table 1 presents various potential pharmacological agents against senescent macrophages. Navitoclax (ABT-263), in combination with D + Q, has been shown to alleviate pulmonary inflammation induced by COVID-19 in mouse models, through the targeted clearance of senescent cells, including macrophages [149]. Navitoclax, a selective inhibitor targeting the anti-apoptotic protein BCL-2, has been shown to improve the phagocytic capacity of macrophages by upregulating the Trem-2 receptor. Furthermore, the drug induces berlin-1-dependent autophagy, significantly reducing sepsis in aged mice [150]. The tyrosine kinase inhibitor D + Q, the earliest identified senolytic drug, effectively eliminates most senescent cells, including macrophages. Medical research has extensively examined the formulation to explore its potential therapeutic applications [109, 151]. Fisetin, a BCL-2 inhibitor, has demonstrated the ability to suppress LPS-induced macrophage activation. Furthermore, Fisetin inhibits ox-LDL-induced senescence in RAW264.7 macrophages via the CKIP-1/REGγ signaling pathway [152, 153]. Nicotinic acid (vitamin B3) and its metabolites have been shown to significantly suppress the generation of ROS, NO, and nitric oxide synthase 2 (NOS2) in LPS-stimulated human macrophages. These compounds also enhance the phagocytic activity of microglia for myelin by upregulating CD36 expression in macrophages [154, 155]. Ganciclovir and D + Q have been shown to restore the phagocytic capacity of irradiated, splenic-derived macrophages by eliminating senescent cells [156]. Cystamine treatment of cultured macrophages has been shown to significantly decrease the production of ROS. Moreover, the compound attenuates macrophage senescence by inhibiting lysosomal oxidation of low-density lipoprotein (LDL) in human macrophages. Additionally, cystamine inhibits the production of TNF-α, IL-6, and MCP-1 by sMACs, thereby markedly reducing the incidence of atherosclerosis in aged mice [157–160]. Selective induction of apoptosis in senescent-like CAFs using the FOXO4-p53 interfering peptide, FOXO4-DRI, enhances the sensitivity of non-small cell lung cancer to radiotherapy and reduces radiation-induced pulmonary fibrosis. FOXO4-DR peptide avoids direct inhibition of BCL-XL, thereby sparing platelets from toxicity [161]. While some anti-aging drugs have already been applied in clinical settings, further research is needed to explore their effects on sMACs and the impact of clearing these cells on tumor progression. Additionally, it is essential to assess the implications of senolytic-mediated clearance of sMACs on tumor biology and progression.
Table 1.
Potential therapeutic agents for senescent macrophages and their mechanisms.
| Category | Drugs | Mechanism | Clinical Trials | Citations |
|---|---|---|---|---|
| Senolytics | Navitoclax | Increases the phagocytic ability of macrophages by upregulating the Trem-2 receptor and blocking the interaction between BCL-2 and beclin-1, thereby inducing beclin-1-dependent autophagy. | [150] | |
| Senolytics | Dasastinib and quercetin (D + Q) | Inhibits multiple tyrosine kinases (such as Src, ABL, c-Kit, etc.) and induces apoptosis. | [109] | |
| Senolytics | Fisetin | Inhibits ox-LDL-induced senescence in RAW264.7 macrophages through the CKIP-1/REGγ pathway. Inhibiting Bcl-2 family proteins promotes apoptosis of senescent cells. | [153] | |
| Senolytics | Niacin (Vitamin B3) | Upregulates the expression of CD36 in macrophages, enhancing the phagocytic activity of microglia for myelin. | [155] | |
| Senolytics | Ganciclovir | Eliminates senescent cells, including senescent macrophages. | [156] | |
| Senolytics | Cysteamine | Inhibits the lysosomal oxidation of LDL in human macrophages, reducing macrophage senescence. | [158, 159] | |
| Senolytics | FOXO4-DRI | FOXO4-DRI can bind to FOXO4, prevent its interaction with p53, release p53, and promote apoptosis. | [161] | |
| Senomorphics | Rapamycin | Suppresses pro-inflammatory SASP production at the translational level by downregulating NF-κB activity. | [164] | |
| Senomorphics | Aspirin | Blocks NF-κB to reduce inflammatory cytokines. | [18] | |
| Senomorphics | Metformin | Activates AMPK to suppress mTOR and lower SASP secretion. | [165] |
Senomorphics
In addition to senolytics, a class of therapeutic strategies known as senomorphics has recently garnered attention. Senomorphics aim to modulate the expression of the SASP to reduce its detrimental impact on the tissue microenvironment, without significantly affecting cell cycle arrest itself [162]. These drugs primarily act by intervening in key signaling pathways closely associated with SASP regulation, including p38 MAPK, PI3K/Akt, mTOR, JAK/STAT, and NF-κB. These pathways play essential roles in the induction, maintenance, and amplification of SASP, making them central targets for senomorphic drug intervention [163].
One of the classic senomorphic agents, rapamycin—an inhibitor of the mTOR—has been widely employed in studies targeting cellular senescence. Rapamycin suppresses pro-inflammatory SASP production at the translational level by downregulating NF-κB activity and has been shown to limit the tumor-promoting effects of senescent fibroblasts within the microenvironment of cancers such as prostate cancer [164]. Aspirin blocks NF-κB to reduce inflammatory cytokines, while metformin activates AMPK to suppress mTOR and lower SASP secretion. Together, these agents help alleviate the inflammatory microenvironment associated with cellular senescence [18, 165].
Challenges and limitations of sMACs
Multifaceted immune regulation of the SASP-related sMACs
SASP, the primary substance secreted by aging cells, comprises a series of inflammatory and interleukin factors, suggesting it plays a more complex and multifaceted role. For example, SASP secreted by precancerous hepatocytes after senescence activates the NF-κB signaling pathway, induces the expression of GM-CSF, Arg1, and IL-6, and significantly enhances CD4⁺ T cell-mediated “senescence surveillance” [166, 167]. In the early stage of HCC, these senescent cells can also recruit CCR2⁺ myeloid cells by secreting CCL2 to eliminate potentially malignant cells and delay tumor formation [168]. On the other hand, in prostate cancer, chemokines and cytokines secreted by senescent cells recruit immunosuppressive MDSCs to weaken anti-tumor immunity in the TME [168]. IL-6, IL-8, and MMPs contained in SASP can significantly promote the invasion and metastasis of pancreatic cancer cells [169]. These findings not only suggest that SASP-related sMACs may have individual differences in different histopathologies or tumor types, but also may play multifaceted immune regulatory roles in the occurrence and development of tumors.
In addition, the senescence of other cells in the TME, including stromal cells, and the background of therapeutic senescence due to chemotherapy and radiotherapy interventions, together enhance the complexity of aging phenotypes within the TME. It remains to be elucidated about the synergistic or exclusive relationship between myeloid-originated sMACs and intratumoral induced sMACs, as well as the relationship between “intratumoral total aging pattern” and immune regulation.
Appropriate sMACs research models to establish causality
The role of sMACs in tumors and chronic diseases has attracted much attention, but the current studies on their functions mostly focus on the relationship between expression levels. For example, single-cell RNA sequencing identified a macrophage subset with high expression of p16, or bulk-TCGA transcriptome data analysis showed that sMACs infiltration was positively correlated with poor prognosis of cancers [116, 135]. The development of a specific and controllable sMACs research model is essential for further research to establish a clear causal relationship.
In the construction of sMACs cells in vitro, other stimulation methods of senescent cells can be used for reference, including cell senescence, which can be induced by radiation, chemical induction, or CRISPR-edited p16 or other senescence promoter genes [170]. For example, Kim et al. used ionizing radiation and doxorubicin to induce senescence of human umbilical vein endothelial cells and found that CXCL11 secreted by them promoted breast cancer invasion, and blocking the CXCL11/CXCR3 pathway could attenuate this effect [171].
In vivo studies, p16Ink4a knockout mice (p16-KO) are often used to analyze senescence-related signals, but p16 deficiency limits the normal occurrence of aging and affects functional studies [172]. Therefore, INK-ATTAC and p16-3MR can induce senescent cell depletion in mouse models by specifically eliminating p16-positive senescent cells, which can effectively evaluate their causal role in tissue homeostasis, chronic inflammation, and disease [173]. Recently, Jean-Philippe et al. revealed the role of aging myeloid cells in the development of lung cancer by transplanting the bone marrow of aging mice into young mice, which provided a new idea for the construction of sMACs or other myeloid cell aging models [80].
Challenges in sMACs-specific targeting
Current senolytic and senomorphic strategies face several limitations, with off-target effects being among the most critical concerns [174, 175]. For example, while the Bcl-2 inhibitor Navitoclax exhibits significant senolytic activity in clearing senescent cells, its clinical application is limited by hematological toxicity, primarily including neutropenia and thrombocytopenia [176, 177]. To mitigate these side effects, various strategies have been explored, such as intermittent dosing regimens or individualized dose adjustments to promote platelet recovery. Combination therapy has also been proven effective in reducing monotherapy exposure, such as combining Navitoclax with ruxolitinib or rituximab, which effectively lowers the required dose while maintaining therapeutic efficacy [178]. Furthermore, alternative therapeutic approaches are under development, including the design of PROTAC molecules that specifically degrade Bcl-XL, thus avoiding direct effects on platelets. Additionally, as mentioned earlier, FOXO4-DR peptides represent a novel class of senolytic drugs that protect platelets by not inhibiting Bcl-XL, thereby reducing hematologic toxicity [161]. Recently, a β-galactosidase-triggered galactose-conjugated nanodrug system (nav-Gal) has been developed, which allows for the selective release of Navitoclax in senescent cells, significantly enhancing therapeutic targeting and minimizing systemic toxicity [175].
In addition, senescent cells (positive for p16INK4a and SA-β-Gal) detected in osteoarthritis (OA) synovium may be derived from both fibroblasts and macrophages [179]. After intraperitoneal injection of senescent cells into mice, the researchers found that F4/80-positive macrophages were predominantly clustered around them, and they also expressed p16 and SA-β-Gal [56]. Moreover, the differences in sMACs across species (e.g., murine vs. human sMAC phenotypes) are also unknown. The above factors together increase the difficulty of targeted clearance of sMACs in clinical applications.
Conclusion
With the continuous progress of research technology and the wide application of single-cell multi-omics technology, sMACs have been found in different tumors, but there is still a lack of research on the causality at the mechanistic level. The sMACs contain a series of secreted factors and molecular biomarkers, which not only expand our understanding of the multifaceted functions of sMACs in immune regulation, but also raise questions about how sMACs participate in the construction of balance in tumors (Fig. 4). Although a variety of anti-aging drugs have entered clinical trials, whether these drugs are effective in sMACs remains to be evaluated. Given that specific targets of sMACs have not yet been developed, finding strategies similar to PD1/PDL1 intervention to reverse T cell exhaustion may be a potential direction for its future clinical applications.
Fig. 4. Intervention strategies of the sMACs iceberg.
The infiltration of sMACs in tissues is like an iceberg above the water, which can be observed and detected. The correlation between the sMACs and the phenotypes and secreted factors, with the intercellular interaction and the formation of TME, with the aging-related diseases and carcinogenesis, like the underwater iceberg, still needs to be researched. The underlying factors at different levels should also be considered in the design of future clinical trials of sMACs-targeted therapies.
Author contributions
WS drafted the original manuscript. YH drafted the original manuscript. XY drafted the original manuscript. YZ designed figures and helped write the manuscript. YP designed figures and helped write the manuscript. YX designed figures and helped write the manuscript. JW designed figures and helped write the manuscript. CW helped write the manuscript. WM revised the manuscript. AZ reviewed and edited the manuscript with input from all authors.
Funding
This work was supported by the National Natural Science Foundation of China (82172567), the Key R&D Plan of Jiangxi Province (2021BBG71006), and the Key Project of Science and Technology Innovation of Health Commission of Jiangxi Province (2023ZD005 and 2024ZD008).
Competing interests
The authors declare no competing interests.
Footnotes
Edited by Francesca Pentimalli
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wenhui Shen, Yueyu Huang, Xuping Yang.
Contributor Information
Weimin Mao, Email: maowm@zjcc.org.cn.
An Zhao, Email: zhaoan@zjcc.org.cn.
References
- 1.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–96. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Gualda E, Baker AG, Fruk L, Munoz-Espin D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021;288:56–80. [DOI] [PubMed] [Google Scholar]
- 3.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. Cellular senescence: defining a path forward. Cell. 2019;179:813–27. [DOI] [PubMed] [Google Scholar]
- 4.Gabai Y, Assouline B, Ben-Porath I. Senescent stromal cells: roles in the tumor microenvironment. Trends Cancer. 2023;9:28–41. [DOI] [PubMed] [Google Scholar]
- 5.Carroll B, Korolchuk VI. Nutrient sensing, growth and senescence. FEBS J. 2018;285:1948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28:436–53. [DOI] [PubMed] [Google Scholar]
- 7.Lecot P, Alimirah F, Desprez PY, Campisi J, Wiley C. Context-dependent effects of cellular senescence in cancer development. Br J Cancer. 2016;114:1180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtani N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): can it be controlled by senolysis?. Inflamm Regen. 2022;42:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Han J, Elisseeff JH, Demaria M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat Rev Mol Cell Biol. 2024;25:958–78. [DOI] [PubMed] [Google Scholar]
- 10.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. [DOI] [PubMed] [Google Scholar]
- 12.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takasugi M, Yoshida Y, Ohtani N. Cellular senescence and the tumour microenvironment. Mol Oncol. 2022;16:3333–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bejarano L, Jordao MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–59. [DOI] [PubMed] [Google Scholar]
- 15.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt CA, Wang B, Demaria M. Senescence and cancer—role and therapeutic opportunities. Nat Rev Clin Oncol. 2022;19:619–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du M, Sun L, Guo J, Lv H. Macrophages and tumor-associated macrophages in the senescent microenvironment: from immunosuppressive TME to targeted tumor therapy. Pharm Res. 2024;204:107198. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Mi S, Ding M, Li X, Yuan S. Metabolism and polarization regulation of macrophages in the tumor microenvironment. Cancer Lett. 2022;543:215766. [DOI] [PubMed] [Google Scholar]
- 21.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40. [DOI] [PubMed] [Google Scholar]
- 22.Johnson DR, Fernandes G, Douglas SD. Age related decline in cytoplasmic spreading of mouse peritoneal macrophages. Dev Comp Immunol. 1978;2:347–54. [DOI] [PubMed] [Google Scholar]
- 23.Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, et al. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–27. [DOI] [PubMed] [Google Scholar]
- 24.Wong CK, Smith CA, Sakamoto K, Kaminski N, Koff JL, Goldstein DR. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J Immunol. 2017;199:1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Duin D, Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc. 2007;55:1438–44. [DOI] [PubMed] [Google Scholar]
- 26.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–701. [DOI] [PubMed] [Google Scholar]
- 27.De La Fuente M. Changes in the macrophage function with aging. Comp Biochem Physiol A Comp Physiol. 1985;81:935–8. [DOI] [PubMed] [Google Scholar]
- 28.Videla LA, Tapia G, Fernández V. Influence of aging on Kupffer cell respiratory activity in relation to particle phagocytosis and oxidative stress parameters in mouse liver. Redox Rep. 2013;6:155–9. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Lankhorst L, Bernards R. Exploiting senescence for the treatment of cancer. Nat Rev Cancer. 2022;22:340–55. [DOI] [PubMed] [Google Scholar]
- 30.Kolodkin-Gal D, Roitman L, Ovadya Y, Azazmeh N, Assouline B, Schlesinger Y, et al. Senolytic elimination of Cox2-expressing senescent cells inhibits the growth of premalignant pancreatic lesions. Gut. 2022;71:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibon E, Loi F, Cordova LA, Pajarinen J, Lin T, Lu L, et al. Aging affects bone marrow macrophage polarization: relevance to bone healing. Regen Eng Transl Med. 2016;2:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured?. Nutr Rev. 2007;65:173–6. [DOI] [PubMed] [Google Scholar]
- 33.Pattabiraman G, Palasiewicz K, Galvin JP, Ucker DS. Aging-associated dysregulation of homeostatic immune response termination (and not initiation). Aging Cell. 2017;16:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med. 2013;210:2223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, et al. p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging. 2017;9:1867–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonnessen-Murray CA, Frey WD, Rao SG, Shahbandi A, Ungerleider NA, Olayiwola JO, et al. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J Cell Biol. 2019;218:3827–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behmoaras J, Gil J. Similarities and interplay between senescent cells and macrophages. J Cell Biol. 2021;220:e202010162. [DOI] [PMC free article] [PubMed]
- 38.Grosse L, Wagner N, Emelyanov A, Molina C, Lacas-Gervais S, Wagner KD, et al. Defined p16(High) senescent cell types are indispensable for mouse healthspan. Cell Metab. 2020;32:87–99.e6. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–76. [DOI] [PubMed] [Google Scholar]
- 40.Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128:1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu JY, Souroullas GP, Diekman BO, Krishnamurthy J, Hall BM, Sorrentino JA, et al. Cells exhibiting strong p16(INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci USA. 2019;116:2603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao H, Jia Q, Yan L, Chen C, Xing S, Shen D. Quercetin suppresses the progression of atherosclerosis by regulating MST1-mediated autophagy in ox-LDL-induced RAW264.7 macrophage foam cells. Int J Mol Sci. 2019;20:6093. [DOI] [PMC free article] [PubMed]
- 44.Stojiljkovic MR, Ain Q, Bondeva T, Heller R, Schmeer C, Witte OW. Phenotypic and functional differences between senescent and aged murine microglia. Neurobiol Aging. 2019;74:56–69. [DOI] [PubMed] [Google Scholar]
- 45.Gažová I, Lefevre L, Bush SJ, Clohisey S, Arner E, de Hoon M, et al. The transcriptional network that controls growth arrest and macrophage differentiation in the human myeloid leukemia cell line THP-1. Front Cell Dev Biol. 2020;8:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baillie JK, Arner E, Daub C, De Hoon M, Itoh M, Kawaji H, et al. Analysis of the human monocyte-derived macrophage transcriptome and response to lipopolysaccharide provides new insights into genetic aetiology of inflammatory bowel disease. PLoS Genet. 2017;13:e1006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. [DOI] [PubMed] [Google Scholar]
- 48.Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell. 2006;5:187–95. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y, Hu Y, Liu Q, Li X, Li X, Kim CY, et al. Two-dimensional design strategy to construct smart fluorescent probes for the precise tracking of senescence. Angew Chem Int Ed Engl. 2021;60:10756–65. [DOI] [PubMed] [Google Scholar]
- 50.Kopp HG, Hooper AT, Shmelkov SV, Rafii S. Beta-galactosidase staining on bone marrow. The osteoclast pitfall. Histol Histopathol. 2007;22:971–6. [DOI] [PubMed] [Google Scholar]
- 51.Ng PY, McNeely TL, Baker DJ. Untangling senescent and damage-associated microglia in the aging and diseased brain. FEBS J. 2021;290:1326–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Qiu H, Chen S, Li D, Zhao X, Guo M, et al. MicroRNA-7 deficiency ameliorates d-galactose-induced aging in mice by regulating senescence of Kupffer cells. Aging Cell. 2024;23:e14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadhu S, Decker C, Sansbury BE, Marinello M, Seyfried A, Howard J, et al. Radiation-induced macrophage senescence impairs resolution programs and drives cardiovascular inflammation. J Immunol. 2021;207:1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su L, Dong Y, Wang Y, Wang Y, Guan B, Lu Y, et al. Potential role of senescent macrophages in radiation-induced pulmonary fibrosis. Cell Death Dis. 2021;12:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holt DJ, Grainger DW. Senescence and quiescence induced compromised function in cultured macrophages. Biomaterials. 2012;33:7497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging. 2016;8:1294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez Marcos PJ, Zoumpourlis V, Trougakos IP, et al. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging. 2013;5:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kloc M, Uosef A, Subuddhi A, Kubiak JZ, Piprek RP, Ghobrial RM. Giant multinucleated cells in aging and senescence-an abridgement. Biology. 2022;11:1121. [DOI] [PMC free article] [PubMed]
- 59.Lin Y, Li Q, Liang G, Xiao N, Yang J, Yang X, et al. Overview of innate immune cell landscape in liver aging. Int J Mol Sci. 2023;25:181. [DOI] [PMC free article] [PubMed]
- 60.Cuollo L, Antonangeli F, Santoni A, Soriani A. The Senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology. 2020;9:485. [DOI] [PMC free article] [PubMed]
- 61.Olivieri F, Prattichizzo F, Grillari J, Balistreri CR. Cellular senescence and inflammaging in age-related diseases. Mediat Inflamm. 2018;2018:9076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao B, Wu B, Feng N, Zhang X, Zhang X, Wei Y, et al. Aging microenvironment and antitumor immunity for geriatric oncology: the landscape and future implications. J Hematol Oncol. 2023;16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krtolica A, Parrinello S, Lockett S, Desprez P-Y, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci. 2001;98:12072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat Commun. 2019;10:2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ortiz-Montero P, Londoño-Vallejo A, Vernot JP. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal. 2017;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell. 2016;30:533–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X, Wang C, Huang Y, Lv Q, Yu C, Ying J, et al. Abnormal cellular populations shape thymic epithelial tumor heterogeneity and anti-tumor by blocking metabolic interactions in organoids. Adv Sci. 2024;11:e2406653. [DOI] [PMC free article] [PubMed]
- 68.Tang T, Scambler TE, Smallie T, Cunliffe HE, Ross EA, Rosner DR, et al. Macrophage responses to lipopolysaccharide are modulated by a feedback loop involving prostaglandin E(2), dual specificity phosphatase 1 and tristetraprolin. Sci Rep. 2017;7:4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haston S, Gonzalez-Gualda E, Morsli S, Ge J, Reen V, Calderwood A, et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell. 2023;41:1242–60.e6. [DOI] [PubMed] [Google Scholar]
- 70.Mirlekar B. Tumor promoting roles of IL-10, TGF-beta, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022;10:20503121211069012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee YS, Kim SY, Song SJ, Hong HK, Lee Y, Oh BY, et al. Crosstalk between CCL7 and CCR3 promotes metastasis of colon cancer cells via ERK-JNK signaling pathways. Oncotarget. 2016;7:36842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han S, Wang T, Chen Y, Han Z, Guo L, Wu Z, et al. High CCL7 expression is associated with migration, invasion and bone metastasis of non-small cell lung cancer cells. Am J Transl Res. 2019;11:442–52. [PMC free article] [PubMed] [Google Scholar]
- 73.Yao J, Li Y, Wang H. The roles of myeloid cells in aging-related liver diseases. Int J Biol Sci. 2023;19:1564–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Xiao J, Cai J, Li R, Sui X, Zhang J, et al. Single-cell immune profiling of mouse liver aging reveals Cxcl2+ macrophages recruit neutrophils to aggravate liver injury. Hepatology. 2024;79:589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li L, Cui L, Lin P, Liu Z, Bao S, Ma X, et al. Kupffer-cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers. Cell Stem Cell. 2023;30:283–99.e9. [DOI] [PubMed] [Google Scholar]
- 76.Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6:377–82. [DOI] [PubMed] [Google Scholar]
- 77.Jiang Q, Cui L, Nie X, Cai H, Zhang W, Lu X, et al. A single-cell transcriptome atlas characterizes the immune landscape of human testes during aging. Aging Cell. 2025;24:e70032. [DOI] [PMC free article] [PubMed]
- 78.Angarola BL, Sharma S, Katiyar N, Kang HG, Nehar-Belaid D, Park S, et al. Comprehensive single-cell aging atlas of healthy mammary tissues reveals shared epigenomic and transcriptomic signatures of aging and cancer. Nat Aging. 2025;5:122–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Redmer T, Raigel M, Sternberg C, Ziegler R, Probst C, Lindner D, et al. JUN mediates the senescence associated secretory phenotype and immune cell recruitment to prevent prostate cancer progression. Mol Cancer. 2024;23:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park MD, Le Berichel J, Hamon P, Wilk CM, Belabed M, Yatim N, et al. Hematopoietic aging promotes cancer by fueling IL-1α-driven emergency myelopoiesis. Science. 2024;386:eadn0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, Ge S, Liao T, Yuan M, Qian W, Chen Q, et al. Integrative single-cell metabolomics and phenotypic profiling reveals metabolic heterogeneity of cellular oxidation and senescence. Nat Commun. 2025;16:2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Maeyer RPH, Chambers ES. The impact of ageing on monocytes and macrophages. Immunol Lett. 2021;230:1–10. [DOI] [PubMed] [Google Scholar]
- 83.Yamaguchi Y, Kaida K, Suenaga Y, Ishigami A, Kobayashi Y, Nagata K. Age-related dysfunction of p53-regulated phagocytic activity in macrophages. Biochem Biophys Res Commun. 2020;529:462–6. [DOI] [PubMed] [Google Scholar]
- 84.Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. 2014;13:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pluvinage JV, Haney MS, Smith BAH, Sun J, Iram T, Bonanno L, et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature. 2019;568:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–7. [DOI] [PubMed] [Google Scholar]
- 87.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Investig Dermatol. 2001;117:1027–35. [DOI] [PubMed] [Google Scholar]
- 88.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, et al. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am J Pathol. 2003;163:711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herrero C, Marques L, Lloberas J, Celada A. IFN-gamma-dependent transcription of MHC class II IA is impaired in macrophages from aged mice. J Clin Investig. 2001;107:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davila DR, Edwards CK 3rd, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990;4:2906–11. [DOI] [PubMed] [Google Scholar]
- 91.Herrero C, Sebastian C, Marques L, Comalada M, Xaus J, Valledor AF, et al. Immunosenescence of macrophages: reduced MHC class II gene expression. Exp Gerontol. 2002;37:389–94. [DOI] [PubMed] [Google Scholar]
- 92.De Maeyer RPH, van de Merwe RC, Louie R, Bracken OV, Devine OP, Goldstein DR, et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol. 2020;21:615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD(+) metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28:1717–28.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci USA. 2015;112:2876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, et al. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019;20:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clement J, Wong M, Poljak A, Sachdev P, Braidy N. The plasma NAD(+) metabolome is dysregulated in “normal” aging. Rejuvenation Res. 2019;22:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Covarrubias AJ, Kale A, Perrone R, Lopez-Dominguez JA, Pisco AO, Kasler HG, et al. Senescent cells promote tissue NAD(+) decline during ageing via the activation of CD38(+) macrophages. Nat Metab. 2020;2:1265–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duong L, Radley-Crabb HG, Gardner JK, Tomay F, Dye DE, Grounds MD, et al. Macrophage depletion in elderly mice improves response to tumor immunotherapy, increases anti-tumor t cell activity and reduces treatment-induced cachexia. Front Genet. 2018;9:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bianchi-Frias D, Damodarasamy M, Hernandez SA, Gil da Costa RM, Vakar-Lopez F, Coleman IM, et al. The aged microenvironment influences the tumorigenic potential of malignant prostate epithelial cells. Mol Cancer Res. 2019;17:321–31. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Zhao Y, Gao Y, Li Y, Liu M, Xu N, et al. Age-related macrophage alterations are associated with carcinogenesis of colorectal cancer. Carcinogenesis. 2022;43:1039–49. [DOI] [PubMed] [Google Scholar]
- 101.Majewska J, Agrawal A, Mayo A, Roitman L, Chatterjee R, Sekeresova Kralova J, et al. p16-dependent increase of PD-L1 stability regulates immunosurveillance of senescent cells. Nat Cell Biol. 2024;26:1336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 pathway: signaling, cancer, and beyond. Adv Exp Med Biol. 2020;1248:33–59. [DOI] [PubMed] [Google Scholar]
- 103.Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20:209–15. [DOI] [PubMed] [Google Scholar]
- 105.Kornepati AVR, Vadlamudi RK, Curiel TJ. Programmed death ligand 1 signals in cancer cells. Nat Rev Cancer. 2022;22:174–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76:359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–62. [DOI] [PubMed] [Google Scholar]
- 108.Lei Q, Wang D, Sun K, Wang L, Zhang Y. Resistance mechanisms of Anti-PD1/PDL1 therapy in solid tumors. Front Cell Dev Biol. 2020;8:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bi W, Yang M, Shi M, Hou M, Jiang C, Fan G, et al. A comprehensive single-cell RNA transcriptomic analysis identifies a unique SPP1+ macrophages subgroup in aging skeletal muscle. Sci Rep. 2024;14:18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z, et al. Single-cell and spatial analysis reveal interaction of FAP(+) fibroblasts and SPP1(+) macrophages in colorectal cancer. Nat Commun. 2022;13:1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sathe A, Mason K, Grimes SM, Zhou Z, Lau BT, Bai X, et al. Colorectal cancer metastases in the liver establish immunosuppressive spatial networking between tumor-associated SPP1+ macrophages and fibroblasts. Clin Cancer Res. 2023;29:244–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu J, Shen Y, Zeng G, Liang Y, Liao G. SPP1(+) TAM subpopulations in tumor microenvironment promote intravasation and metastasis of head and neck squamous cell carcinoma. Cancer Gene Ther. 2024;31:311–21. [DOI] [PubMed] [Google Scholar]
- 113.Nallasamy P, Nimmakayala RK, Karmakar S, Leon F, Seshacharyulu P, Lakshmanan I, et al. Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1-CD44 axis. Gastroenterology. 2021;161:1998–2013.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hao C, Lane J, Jiang WG. Osteopontin and cancer: insights into its role in drug resistance. Biomedicines. 2023;11:197. [DOI] [PMC free article] [PubMed]
- 115.Matsubara E, Yano H, Pan C, Komohara Y, Fujiwara Y, Zhao S, et al. The significance of SPP1 in lung cancers and its impact as a marker for protumor tumor-associated macrophages. Cancers. 2023;15:2250. [DOI] [PMC free article] [PubMed]
- 116.Prieto LI, Sturmlechner I, Graves SI, Zhang C, Goplen NP, Yi ES, et al. Senescent alveolar macrophages promote early-stage lung tumorigenesis. Cancer Cell. 2023;41:1261–75.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Molczyk C, Singh RK. CXCR1: a cancer stem cell marker and therapeutic target in solid tumors. Biomedicines. 2023;11:576. [DOI] [PMC free article] [PubMed]
- 118.Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7:1543–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–62. [DOI] [PubMed] [Google Scholar]
- 121.Sharma B, Singh S, Varney ML, Singh RK. Targeting CXCR1/CXCR2 receptor antagonism in malignant melanoma. Expert Opin Ther Targets. 2010;14:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwak JW, Nguyen HQ, Camai A, Huffman GM, Mekvanich S, Kenney NN, et al. CXCR1/2 antagonism inhibits neutrophil function and not recruitment in cancer. Oncoimmunology. 2024;13:2384674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ron D, Adams DR, Baillie GS, Long A, O’Connor R, Kiely PA. RACK1 to the future-a historical perspective. Cell Commun Signal. 2013;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen T, Wang F, Wei S, Nie Y, Zheng X, Deng Y, et al. FGFR/RACK1 interacts with MDM2, promotes P53 degradation, and inhibits cell senescence in lung squamous cell carcinoma. Cancer Biol Med. 2021;18:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu Q, Chen L, Li Y, Huang M, Shao J, Li S, et al. Rack1 is essential for corticogenesis by preventing p21-dependent senescence in neural stem cells. Cell Rep. 2021;36:109639. [DOI] [PubMed] [Google Scholar]
- 126.Dan H, Liu S, Liu J, Liu D, Yin F, Wei Z, et al. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF-κB pathway in oral squamous cell carcinoma. Mol Oncol. 2020;14:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu Y, Liu JP, Li XY, Cai Y, He C, Li NS, et al. Downregulation of tumor suppressor RACK1 by Helicobacter pylori infection promotes gastric carcinogenesis through the integrin β-1/NF-κB signaling pathway. Cancer Lett. 2019;450:144–54. [DOI] [PubMed] [Google Scholar]
- 128.Zhang L, Lv Y, Rong Y, Chen W, Fang Y, Mao W, et al. Downregulated expression of RACK1 results in pancreatic cancer growth and metastasis. Onco Targets Ther. 2019;12:1007–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krasniewski LK, Chakraborty P, Cui CY, Mazan-Mamczarz K, Dunn C, Piao Y, et al. Single-cell analysis of skeletal muscle macrophages reveals age-associated functional subpopulations. Elife. 2022;11:e77974. [DOI] [PMC free article] [PubMed]
- 130.Banerji S, Ni J, Wang S-X, Clasper S, Su J, Tammi R, et al. LYVE-1, a New homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carvalho AM, Reis RL, Pashkuleva I. Hyaluronan receptors as mediators and modulators of the tumor microenvironment. Adv Health Mater. 2023;12:e2202118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dollt C, Becker K, Michel J, Melchers S, Weis CA, Schledzewski K, et al. The shedded ectodomain of Lyve-1 expressed on M2-like tumor-associated macrophages inhibits melanoma cell proliferation. Oncotarget. 2017;8:103682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng Z, Wu J. hsa_circ_0129047 upregulates LYVE1 to inhibit hepatocellular carcinoma progression by sponging miR-492. Dis Markers. 2023;2023:6978234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang G, Dong K, Liu J, Zhou W. Prognosis and tumor immune microenvironment of patients with gastric cancer by a novel senescence-related signature. Medicine. 2022;101:e30927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang Q, Zhou J, Chen Q, Huang Y, Yang C, Liu C. Construction and experimental validation of a macrophage cell senescence-related gene signature to evaluate the prognosis, immunotherapeutic sensitivity, and chemotherapy response in bladder cancer. Funct Integr Genom. 2023;23:228. [DOI] [PubMed] [Google Scholar]
- 136.Zhao Z, Hao Z, Zhang Z, Zhan X. Bioinformatics analysis reveals the vital role of AKR1B1 in immune infiltration and clinical outcomes of gastric cancer. DNA Cell Biol. 2023;42:372–89. [DOI] [PubMed] [Google Scholar]
- 137.Khayami R, Hashemi SR, Kerachian MA. Role of aldo-keto reductase family 1 member B1 (AKR1B1) in the cancer process and its therapeutic potential. J Cell Mol Med. 2020;24:8890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Di Benedetto C, Borini Etichetti C, Cocordano N, Cantoia A, Arel Zalazar E, Bicciato S, et al. The p53 tumor suppressor regulates AKR1B1 expression, a metastasis-promoting gene in breast cancer. Front Mol Biosci. 2023;10:1145279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang KR, Zhang YF, Lei HM, Tang YB, Ma CS, Lv QM, et al. Targeting AKR1B1 inhibits glutathione de novo synthesis to overcome acquired resistance to EGFR-targeted therapy in lung cancer. Sci Transl Med. 2021;13:eabg6428. [DOI] [PubMed] [Google Scholar]
- 140.Yadav UC, Ramana KV, Srivastava SK. Aldose reductase inhibition suppresses airway inflammation. Chem Biol Interact. 2011;191:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288:518–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995;55:2284–92. [PubMed] [Google Scholar]
- 144.Robbins PD, Jurk D, Khosla S, Kirkland JL, LeBrasseur NK, Miller JD, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharm Toxicol. 2021;61:779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Novais EJ, Tran VA, Johnston SN, Darris KR, Roupas AJ, Sessions GA, et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12:5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhu Y, Prata L, Gerdes EOW, Netto JME, Pirtskhalava T, Giorgadze N, et al. Orally-active, clinically-translatable senolytics restore alpha-Klotho in mice and humans. EBioMedicine. 2022;77:103912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kashyap D, Garg VK, Tuli HS, Yerer MB, Sak K, Sharma AK, et al. Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules. 2019;9:174. [DOI] [PMC free article] [PubMed]
- 149.Lee S, Yu Y, Trimpert J, Benthani F, Mairhofer M, Richter-Pechanska P, et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature. 2021;599:283–9. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y, Tang LH, Lu J, Xu LM, Cheng BL, Xiong JY. ABT-263 enhanced bacterial phagocytosis of macrophages in aged mouse through Beclin-1-dependent autophagy. BMC Geriatr. 2021;21:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saccon TD, Nagpal R, Yadav H, Cavalcante MB, Nunes ADdC, Schneider A, et al. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J Gerontol Ser A. 2021;76:1895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu SH, Lin CH, Hung SK, Chou JH, Chi CW, Fu SL. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J Agric Food Chem. 2010;58:10831–9. [DOI] [PubMed] [Google Scholar]
- 153.Jia Q, Cao H, Shen D, Yan L, Chen C, Xing S. Fisetin, via CKIP-1/REGgamma, limits oxidized LDL-induced lipid accumulation and senescence in RAW264.7 macrophage-derived foam cells. Eur J Pharm. 2019;865:172748. [DOI] [PubMed] [Google Scholar]
- 154.Montserrat-de la Paz S, Naranjo MC, Lopez S, Abia R, Muriana FJG, Bermudez B. Niacin and its metabolites as master regulators of macrophage activation. J Nutr Biochem. 2017;39:40–7. [DOI] [PubMed] [Google Scholar]
- 155.Rawji KS, Young AMH, Ghosh T, Michaels NJ, Mirzaei R, Kappen J, et al. Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol. 2020;139:893–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palacio L, Goyer ML, Maggiorani D, Espinosa A, Villeneuve N, Bourbonnais S, et al. Restored immune cell functions upon clearance of senescence in the irradiated splenic environment. Aging Cell. 2019;18:e12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Okamura DM, Bahrami NM, Ren S, Pasichnyk K, Williams JM, Gangoiti JA, et al. Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD. J Am Soc Nephrol. 2014;25:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ahmad F, Leake DS. Lysosomal oxidation of LDL alters lysosomal pH, induces senescence, and increases secretion of pro-inflammatory cytokines in human macrophages. J Lipid Res. 2019;60:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wen Y, Ahmad F, Mohri Z, Weinberg PD, Leake DS. Cysteamine inhibits lysosomal oxidation of low density lipoprotein in human macrophages and reduces atherosclerosis in mice. Atherosclerosis. 2019;291:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ahmad F, Mitchell RD, Houben T, Palo A, Yadati T, Parnell AJ, et al. Cysteamine decreases low-density lipoprotein oxidation, causes regression of atherosclerosis, and improves liver and muscle function in low-density lipoprotein receptor-deficient mice. J Am Heart Assoc. 2021;10:e017524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meng J, Li Y, Wan C, Sun Y, Dai X, Huang J, et al. Targeting senescence-like fibroblasts radiosensitizes non-small cell lung cancer and reduces radiation-induced pulmonary fibrosis. JCI Insight. 2021;6:e146334. [DOI] [PMC free article] [PubMed]
- 162.Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ, Robbins PD. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. Febs j. 2023;290:1362–83. [DOI] [PubMed] [Google Scholar]
- 163.Xiao J, Li HS, Satyanarayanan SK, Leung SL, Yuan Q, Wang Y, et al. Advancements in targeting macrophage senescence for age-associated conditions. Aging Dis. 2024;16:2201–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang H, Xu Q, Jiang Z, Sun R, Wang Q, Liu S, et al. Targeting senescence with apigenin improves chemotherapeutic efficacy and ameliorates age-related conditions in mice. Adv Sci (Weinh). 2025;12:e2412950. [DOI] [PMC free article] [PubMed]
- 166.Gu M, Liu Y, Zheng W, Jing Z, Li X, Guo W, et al. Combined targeting of senescent cells and senescent macrophages: a new idea for integrated treatment of lung cancer. Semin Cancer Biol. 2024;106-107:43–57. [DOI] [PubMed] [Google Scholar]
- 167.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. [DOI] [PubMed] [Google Scholar]
- 168.Takasugi M, Yoshida Y, Hara E, Ohtani N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 2023;290:1348–61. [DOI] [PubMed] [Google Scholar]
- 169.Ruhland MK, Loza AJ, Capietto AH, Luo X, Knolhoff BL, Flanagan KC, et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Commun. 2016;7:11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.He Y, Qiu Y, Yang X, Lu G, Zhao SS. Remodeling of tumor microenvironment by cellular senescence and immunosenescence in cervical cancer. Semin Cancer Biol. 2025;108:17–32. [DOI] [PubMed] [Google Scholar]
- 171.Hwang HJ, Lee YR, Kang D, Lee HC, Seo HR, Ryu JK, et al. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Cancer Lett. 2020;490:100–10. [DOI] [PubMed] [Google Scholar]
- 172.Baker DJ, Perez-Terzic C, Jin F, Pitel KS, Niederländer NJ, Jeganathan K, et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol. 2008;10:825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Grigorash B, Bulavin DV. Depletion of p16(high) senescent cells for stem cell reprogramming and tissue rejuvenation. Nat Cell Biol. 2023;25:1252–1253. [DOI] [PubMed] [Google Scholar]
- 174.Power H, Valtchev P, Dehghani F, Schindeler A. Strategies for senolytic drug discovery. Aging Cell. 2023;22:e13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang L, Tang D. Immunosenescence promotes cancer development: from mechanisms to treatment strategies. Cell Commun Signal. 2025;23:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xiong J, Dong L, Lv Q, Yin Y, Zhao J, Ke Y, et al. Targeting senescence-associated secretory phenotypes to remodel the tumour microenvironment and modulate tumour outcomes. Clin Transl Med. 2024;14:e1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Patel M, Potluri J, Marbury T, Lawitz E, Rondon JC, Hoffman DM, et al. Pharmacokinetics and safety of navitoclax in hepatic impairment. Clin Pharmacokinet. 2025;64:611–7. [DOI] [PubMed] [Google Scholar]
- 179.Liu Y, Zhang Z, Li T, Xu H, Zhang H. Senescence in osteoarthritis: from mechanism to potential treatment. Arthritis Res Ther. 2022;24:174. [DOI] [PMC free article] [PubMed] [Google Scholar]