Abstract
1. Repetitive intracellular stimulation at a frequency of 5-30 Hz for 1-10 s evoked in neurones of the isolated inferior mesenteric and superior cervical ganglia of the guinea-pig three types of post-spike membrane potential changes: (i) hyperpolarization, (ii) hyperpolarization followed by a slow depolarization, and (iii) a second hyperpolarization following the initial two responses.
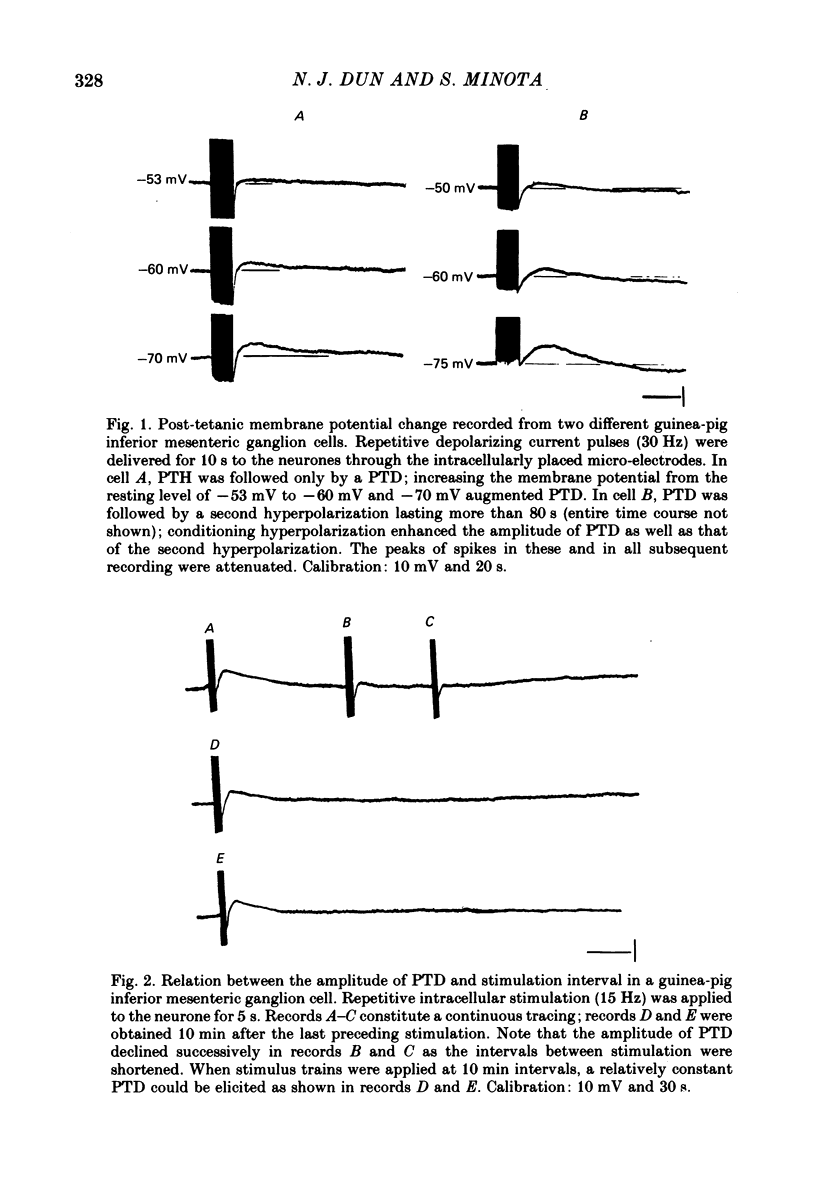
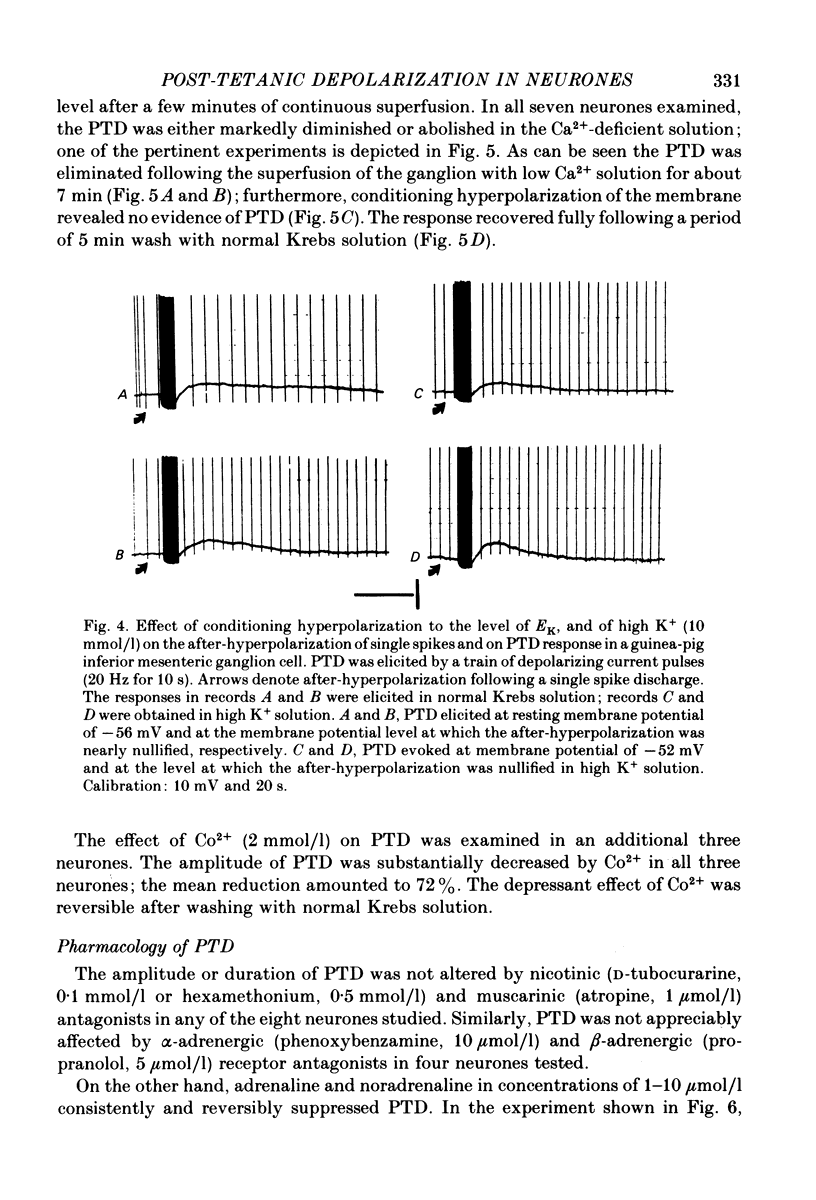
2. The initial post-spike hyperpolarization had a mean duration of 2·0 s and was often associated with a fall in membrane resistance; it could be elicited in every sympathetic neurone studied. This response was termed the post-tetanic hyperpolarization (PTH).
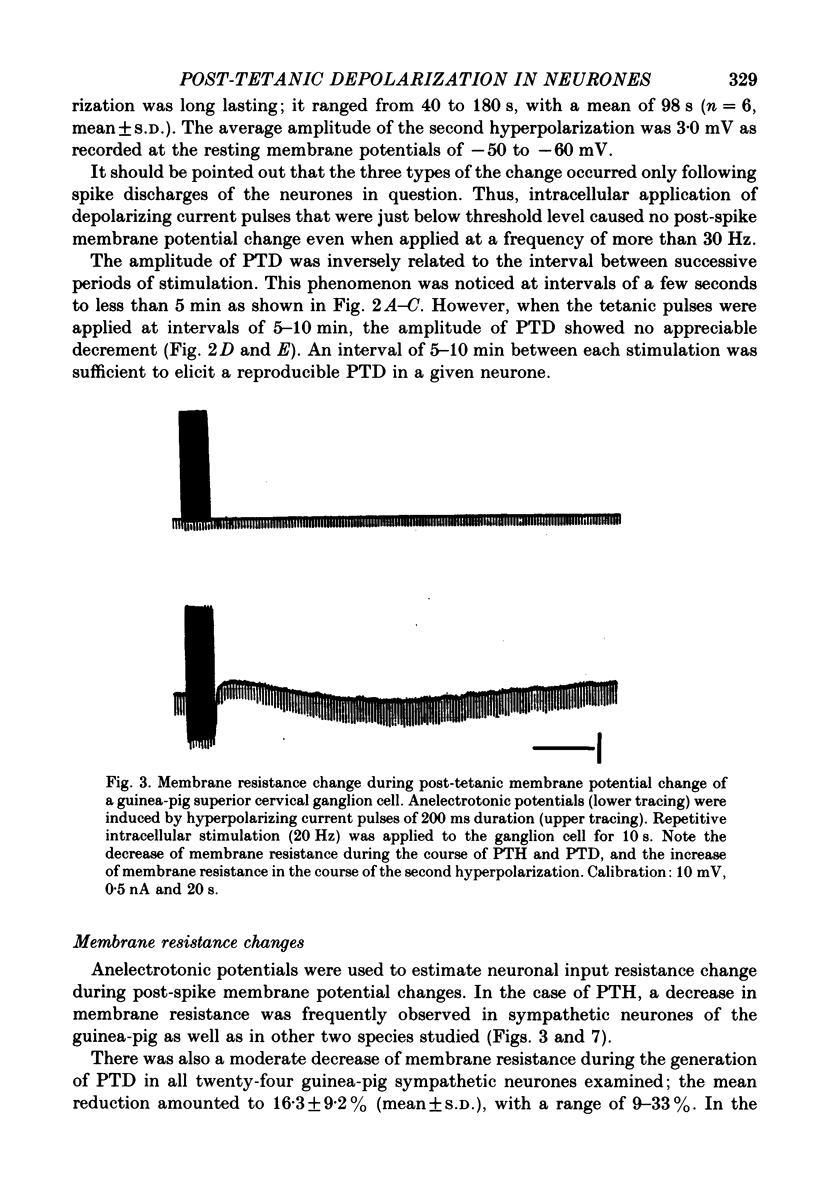
3. The slow depolarization which could be induced only in a portion of neurones had a mean amplitude and duration of 2·2 mV and 27·5 s, respectively; it was termed the post-tetanic depolarization (PTD).
4. PTD was associated with a fall in membrane resistance, augmented by membrane hyperpolarization, and reduced by depolarization; its mean extrapolated equilibrium potential was -38 mV.
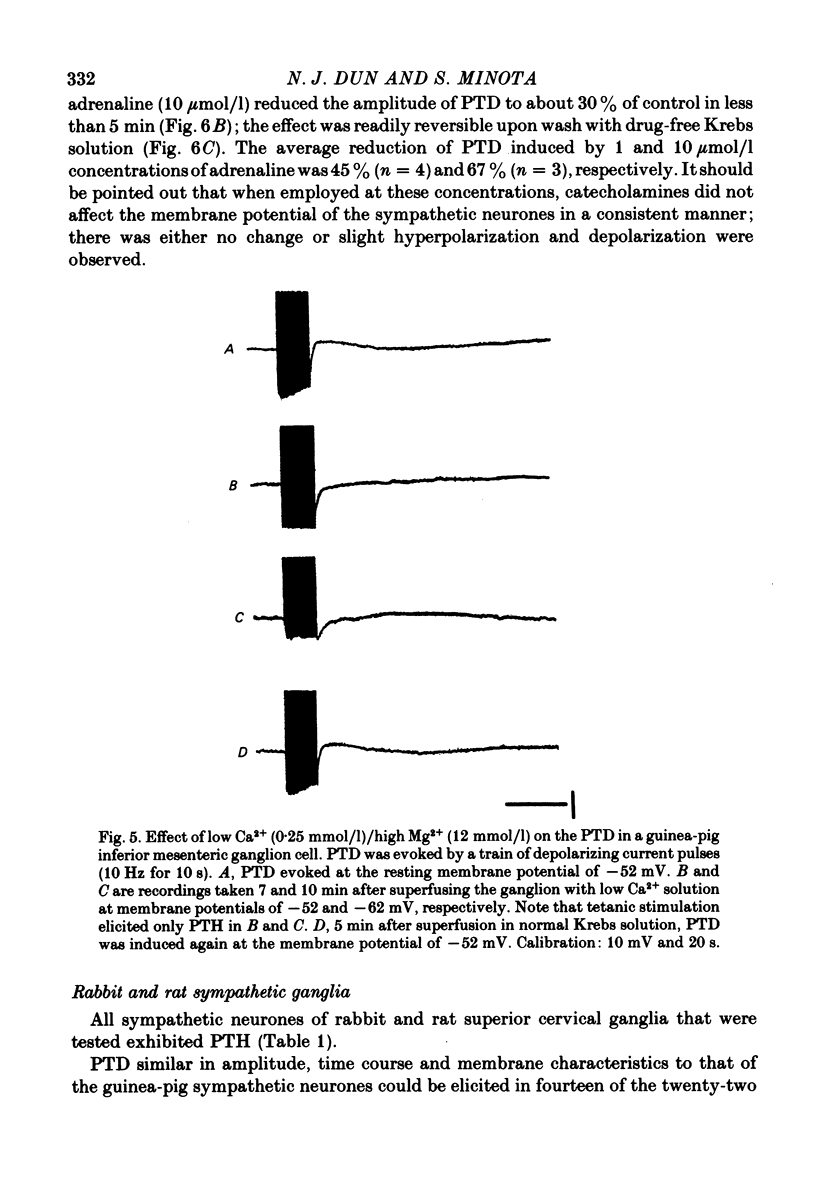
5. PTD was not blocked by nicotinic and muscarinic antagonists, or α-and β-adrenergic receptor antagonists, whereas it was suppressed by adrenaline, noradrenaline, Co2+ and a low Ca2+ solution.
6. The amplitude of the single spike after-hyperpolarization in normal Krebs solution as well as in high K+ solution was increased during PTD; furthermore, conditioning hyperpolarization to the level of EK increased the amplitude of PTD in normal Krebs as well as in high K+ solution.
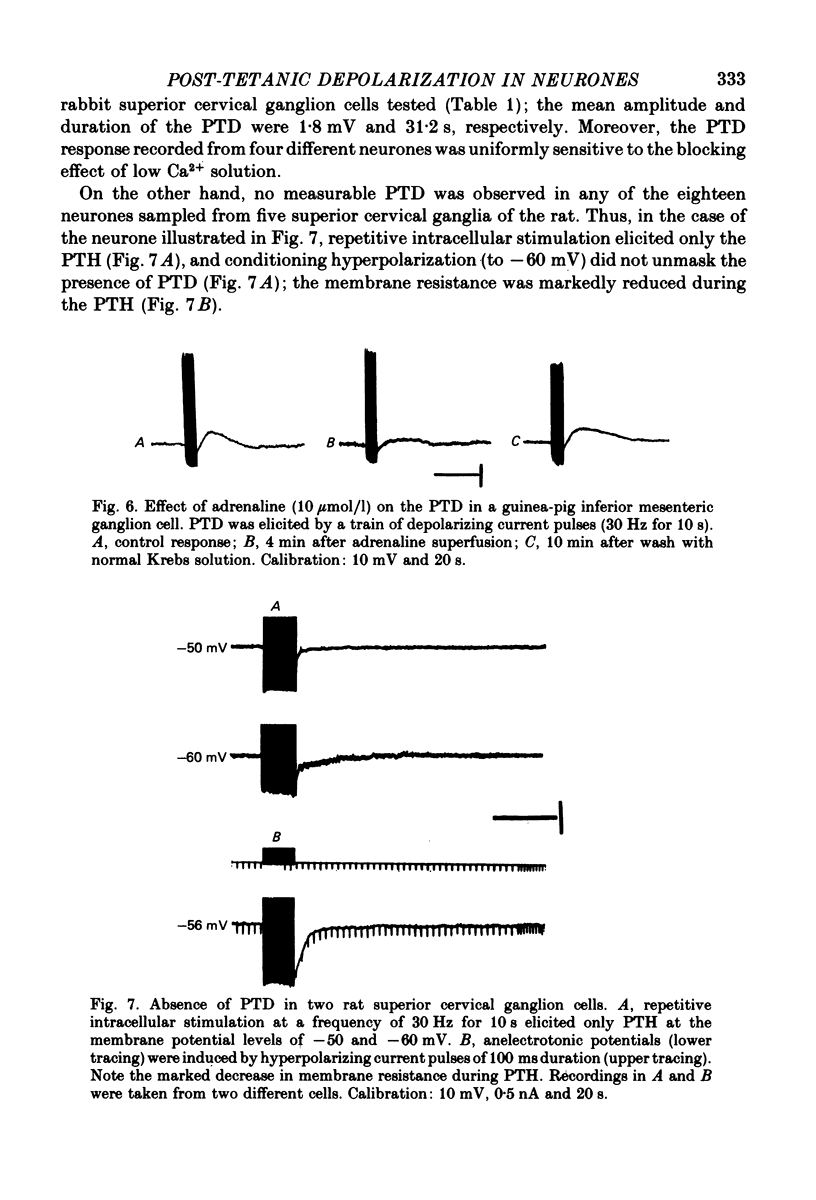
7. PTD with similar amplitude, time course and membrane characteristics could be evoked in a portion of neurones of the rabbit superior cervical ganglia; however, PTD was not detected in neurones of the rat superior cervical ganglia.
8. Decentralization of the guinea-pig and rabbit superior cervical ganglia for 14 d did not alter the number of neurones in which PTD could be elicited, its amplitude, or its time course.
9. Our results suggest that a chemical substance(s) is responsible for the generation of PTD; it may be released from the soma and/or dendrites and acts in an auto-receptive manner on the cells in question. The nature and origin of the second hyperpolarization remain to be clarified.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Nicholls J. G. After-effects of nerve impulses on signalling in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):571–589. doi: 10.1113/jphysiol.1969.sp008880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodwick M. S., Junge D. Post-stimulus hyperpolarization and slow potassium conductance increase in Aplysia giant neurone. J Physiol. 1972 Jun;223(2):549–570. doi: 10.1113/jphysiol.1972.sp009862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ D. D., Nishi S. Site of adrenaline blockade in the superior cervical ganglion of the rabbit. J Physiol. 1971 Feb;213(1):107–117. doi: 10.1113/jphysiol.1971.sp009371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J. Ganglionic transmission: electrophysiology and pharmacology. Fed Proc. 1980 Oct;39(12):2982–2989. [PubMed] [Google Scholar]
- Dun N., Karczmar A. G. The presynaptic site of action of norepinephrine in the superior cervical ganglion of guinea pig. J Pharmacol Exp Ther. 1977 Feb;200(2):328–335. [PubMed] [Google Scholar]
- Dun N., Nishi S. Effects of dopamine on the superior cervical ganglion of the rabbit. J Physiol. 1974 May;239(1):155–164. doi: 10.1113/jphysiol.1974.sp010560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N., Nishi S., Karczmar A. G. Alteration in nicotinic and muscarinic responses of rabbit superior cervical ganglion cells after chronic preganglionic denervation. Neuropharmacology. 1976 Apr;15(4):211–218. doi: 10.1016/0028-3908(76)90066-6. [DOI] [PubMed] [Google Scholar]
- Erulkar S. D., Weight F. F. Extracellular potassium and trasmitter release at the giant synapse of squid. J Physiol. 1977 Apr;266(2):209–218. doi: 10.1113/jphysiol.1977.sp011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen L. B., Jessell T. M., Cuello A. C., Iversen L. L. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976 Mar 18;260(5548):258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Elde R., Schultzberg M., Goldstein M., Luft R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3587–3591. doi: 10.1073/pnas.74.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. J Physiol. 1973 Mar;229(3):635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K. The electrogenic sodium pump. Adv Biophys. 1971;2:77–112. [PubMed] [Google Scholar]
- Kondo H., Dun N. J., Pappas G. D. A light and electron microscopic study of the rat superior cervical ganglion cells by intracellular HRP-labeling. Brain Res. 1980 Sep 15;197(1):193–199. doi: 10.1016/0006-8993(80)90444-8. [DOI] [PubMed] [Google Scholar]
- Korf J., Zieleman M., Westerink B. H. Dopamine release in substantia nigra? Nature. 1976 Mar 18;260(5548):257–258. doi: 10.1038/260257a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T., Weakly J. N. Post-tetanic hyperpolarization produced by an electrogenic pump in dorsal spinocerebellar tract neurones of the cat. J Physiol. 1970 Nov;210(4):839–855. doi: 10.1113/jphysiol.1970.sp009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Schultzberg M., Uvnäs-Wallensten K., Köhler C., Said S. I. Occurrence of vasoactive intestinal polypeptide (VIP)-like immunoreactivity in certain cholinergic neurons of the cat: evidence from combined immunohistochemistry and acetylcholinesterase staining. Neuroscience. 1979;4(11):1539–1559. doi: 10.1016/0306-4522(79)90018-6. [DOI] [PubMed] [Google Scholar]
- Minota S. Calcium ions and the post-tetanic hyperpolarization of bullfrog sympathetic ganglion cells. Jpn J Physiol. 1974 Oct;24(5):501–512. doi: 10.2170/jjphysiol.24.501. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]


