Abstract
Aminoacyl-tRNA synthetases have attracted interest as essential and novel targets involved in bacterial protein synthesis. REP8839 is a potent inhibitor of MetS, the methionyl-tRNA synthetase in Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), and in Streptococcus pyogenes. The biochemical activity of REP8839 was shown by specific inhibition of purified S. aureus MetS (50% inhibitory concentration, <1.9 nM). Target specificity was confirmed by overexpression of the metS gene in S. aureus, resulting in an eightfold increase in the MIC for REP8839. Macromolecular synthesis assays in the presence of REP8839 demonstrated a dose-dependent inhibition of protein synthesis and RNA synthesis in S. pneumoniae R6, but only protein synthesis was affected in an isogenic rel mutant deficient in the stringent response. Strains with reduced susceptibility to REP8839 were generated by selection of strains with spontaneous mutations and through serial passages. Point mutations within the metS gene were mapped, leading to a total of 23 different amino acid substitutions within MetS that were located around the modeled active site. The most frequent MetS mutations were I57N, leading to a shift in the MIC from 0.06 μg/ml to 4 μg/ml, and G54S, resulting in a MIC of 32 μg/ml that was associated with a reduced growth rate. The mutation prevention concentration was 32 μg/ml in four S. aureus strains (methicillin-sensitive S. aureus and MRSA), which is well below the drug concentration of 2% (20,000 μg/ml) in a topical formulation. In conclusion, we demonstrate by biochemical, physiologic, and genetic mode-of-action studies that REP8839 exerts its antibacterial activity through specific inhibition of MetS, a novel target.
Methicillin-resistant Staphylococcus aureus (MRSA) is a major cause of nosocomial infections with severe morbidity and mortality worldwide. According to a recent surveillance study, the methicillin (oxacillin) resistance rates among intensive care unit S. aureus isolates from the United States, Canada, and Europe ranged from 19.7% to 59.4% (29). In the United States, 42.8% of all S. aureus isolates in 2003 were methicillin resistant. In the United Kingdom, the MRSA rate has increased by 5% from 2003 to 2004 to reach 40% (50). MRSA outbreaks in the community are also on a sharp incline. This rising global health and socioeconomic problem demands new measures for prevention and control of MRSA (11, 41, 46). Efforts have been made to decrease nosocomial MRSA outbreaks through identification of MRSA colonization at hospital admission followed by adequate interventions (9). Since the elimination of nasal carriage could prevent infection, decolonization of carriers, especially in high-risk groups, is an important goal and warrants the discovery and development of new remedies (33). Many different topical agents have the potential to eliminate MRSA from the anterior nares or from the skin of carriers and thereby decrease the risk of subsequent infection. These include mupirocin, known as Bactroban ointment (32, 39), fusidic acid (36), indolmycin (23), silver sulfadiazine with cerium nitrate (43), chlorhexidine (51), tea tree oil (4), garlic-derived allicin extract (8), and autolysins, such as lysostaphin (31). Mupirocin, an inhibitor of isoleucyl-tRNA synthetase (IRS), has been used for many years as a topical agent to eliminate nasal carriage of S. aureus and to treat community-acquired skin infections, such as impetigo and secondarily infected traumatic skin lesions due to staphylococci and streptococci (21). However, resistance to mupirocin is on the rise, and its effectiveness is compromised in areas where clinical use is high, leading to persistence or recolonization (44, 47). According to the SENTRY antimicrobial surveillance program in 2000, 1.9 to 5.6% of S. aureus isolates and 12.8 to 39.9% of coagulase-negative staphylococci isolated in the United States, Canada, Latin America, and Europe were mupirocin resistant (10). Both low-level mupirocin resistance (MIC = 8 to 256 μg/ml) and high-level resistance (MIC > 256 μg/ml) have been described elsewhere (13, 15). Low-level mupirocin resistance is caused by point mutations which have been mapped within the ileS gene encoding the isoleucyl-tRNA synthetase (1). High-level mupirocin resistance is mediated through acquisition of a second and phylogenetically distinct isoleucyl-tRNA synthetase gene, mupA (18).
Aminoacyl-tRNA synthetases carry out the condensation of a specific amino acid with its cognate tRNA in a reaction that is dependent on ATP. Methionyl-tRNA synthetase (MRS) represents a novel target that is essential and well conserved among gram-positive microbes. Most bacteria, including S. aureus, contain a type 1 MRS, although a significant proportion of Streptococcus pneumoniae strains contain a second gene, metS2, which encodes a type 2 MRS that is more closely related to archaeal synthetases (3, 14). Both types of MRS belong to class I tRNA synthetases that harbor an ATP-binding Rossman fold with the conserved H(M/I)GH and KMSKS motifs (1, 12). A potent MRS type 1 inhibitor with antibacterial activity against staphylococci and enterococci has recently been identified as a high-throughput screening hit (26). Subsequent medicinal chemistry efforts resulted in REP8839, which is a fluorovinylthiophene-containing diaryldiamine with a promising antibacterial profile and spectrum of activity (7, 17, 25, 27, 28). Here, we report on the mode of action of REP8839, including the characterization of laboratory-generated mutants with decreased susceptibility to this novel synthetic compound.
MATERIALS AND METHODS
Strains, plasmids, media, and chemicals.
S. aureus and Streptococcus pyogenes strains were from the American Type Culture Collection (ATCC) (Manassas, VA), from Focus Bio-Inova (Herndon, VA) and NARSA (Network on Antimicrobial Resistance in S. aureus; Focus Bio-Inova). S. aureus strain RN4220 containing pYH4-metS and the S. pneumoniae strains R6 and R6 rel (16) were from GlaxoSmithKline (Collegeville, PA). Mueller-Hinton broth (MHB), Mueller-Hinton agar (MHA), and blood agar plates were from Remel (Lenexa, KS). Streptococci were grown in MHB containing 3% lysed horse blood. Purified preparations of S. aureus MetS, Haemophilus influenzae MetG, Escherichia coli MetG, and S. pneumoniae MetS2, as well as a rat liver lysate, were provided by GlaxoSmithKline (Collegeville, PA). E. coli MRE 600 tRNA was from Roche Applied Science (Indianapolis, IN).
REP8839 was synthesized at Replidyne, and mupirocin was from Pliva (Zagreb, Croatia). Novobiocin (USP), gentamicin (Sigma), and vancomycin (USP) were used as control agents. Anhydrotetracycline was from BD Biosciences (Palo Alto, CA). The radiolabeled compounds [5-3H]uridine, l-[4,5-3H]leucine, and l-[methyl-3H]methionine were from Amersham Biosciences Corp. (Piscataway, NJ).
MRS enzymatic assay.
Assays for inhibition of tRNAMet aminoacylation were carried out much as described previously (34). Reaction mixtures (50 μl) contained 65 mM Tris-HCl, pH 8.0, 80 mM KCl, 10 mM magnesium acetate, 2.5 mM dithiothreitol, 5 mM ATP, 1 mg/ml E. coli tRNA, 0.25 mg/ml bovine serum albumin, and 7 μM methionine (1.4 μCi/nmol) in round-bottom 96-well Costar plates (Corning, NY). MRS enzymes were diluted in 50 mM Tris-HCl, pH 8.0, 2 mM dithiothreitol, and 0.3 mg/ml bovine serum albumin and added (26 μl) to wells containing REP8839 (4 μl) in dimethyl sulfoxide or to control wells containing 4 μl dimethyl sulfoxide or 0.5 M EDTA. A cofactor mix containing the remaining reaction mixture components (20 μl) was added, and plates were incubated for 15 min at room temperature (23°C). Reactions were terminated, and the tRNA was precipitated by the addition of 150 μl 5% trichloroacetic acid (TCA). Reaction mixtures were transferred to 96-well filter plates (Durapore) (catalog no. MVHBN4550; Millipore, Bedford, MA) and filtered using a Manifold (Innovative Microplate, Chicopee, MA). Samples were washed with 300 μl 10% trichloroacetic acid, followed by 300 μl 95% ethanol, and air dried overnight. Reaction products were counted by liquid scintillation using MicroScint (50 μl) and a TopCount-NXT (Packard BioScience, Boston, MA).
Microbiological assays.
Broth microdilution MIC testing was performed in 96-well microtiter plates according to CLSI (formerly NCCLS) document M7-A6 (6). MIC testing of the metS overexpressor strain S. aureus RN4220(pYH4-metS) occurred in the presence or absence of anhydrotetracycline (10 ng/ml) to regulate metS expression from the TetR-dependent promoter. Macromolecular synthesis assays were performed in S. pneumoniae R6 and an isogenic rel mutant (16). The cells were grown statically for 6 h at 35°C in 10 ml MHB containing 3% lysed horse blood, and the cell density was adjusted to match the 0.5 McFarland standard (approximately 108 CFU/ml). A 96-well microtiter plate containing 50 μl REP8839 (0.008 to 16 μg/ml) was inoculated with 50 μl of cells and incubated for 10 min at 35°C, and the radiolabeled precursors [5-3H]uridine and l-[4,5-3H]leucine were added. Incorporation was stopped after 10 min by the addition of 100 μl of 20% ice-cold TCA. The plates were refrigerated for 1 h to allow cell lysis and precipitation of macromolecules, followed by vacuum filtration of the samples through 96-well Durapore plates (Millipore, Bedford, MA). The filter plates were washed twice with 200 μl of 10% ice-cold TCA and then once with 200 μl of cold ethanol and air dried. MicroScint O (Perkin-Elmer, Boston, MA) was added (50 μl), the plates were sealed and counted in a Packard TopCount NXT, and the data were normalized to the data for untreated controls. Growth competition assays between S. aureus wild-type cells and MetS mutant cells were performed in cocultures with a starting inoculum of roughly 105 CFU/ml of each strain. The cultures were grown at 35°C with shaking (200 rpm), and samples were removed at hourly intervals. Serial 10-fold dilutions in saline were prepared in a 96-well plate, and 10 μl of each dilution was spotted on blood agar (total CFU) and on selective agar containing 1 μg/ml of REP8839 (MetS mutant CFU). Colonies were enumerated, and wild-type CFU were calculated. Additionally, the ratios of wild-type to mutant cells were calculated in late exponential growth phase (9.5 h) and in stationary phase (24 h).
Spontaneous resistance rates and MPC determination.
S. aureus cells were grown in MHB for 4 to 6 h at 35°C, harvested by centrifugation (4,000 rpm, 10 min) and resuspended in MHB at about 1010 CFU/ml. Agar plates containing REP8839 or mupirocin (1 to 32 μg/ml) were seeded with 0.1 ml of this cell suspension and incubated at 35°C. Colonies were counted after 48 h, and the spontaneous resistance rates were calculated on the basis of the exact cell number in the inoculum, as determined on drug-free plates. These first-step mutants were purified on MHA containing REP8839 or mupirocin at half the original concentration. Second-step mutants were isolated by repeating the procedure above with first-step isolates. Mutant stability was assessed by MIC testing after serial propagation on five drug-free plates. To determine the mutation prevention concentration (MPC), five agar plates at each drug concentration were seeded each with 2 × 109 CFU to achieve an inoculum of 1010 CFU, and colonies were counted after 5 days.
Serial passages.
Changes in the susceptibilities of bacteria to REP8839 were monitored during serial passages in drug-containing broth. The first passage was a broth microdilution MIC test. For the subsequent passages, the cells growing in the well with the highest inhibitor concentration, typically 0.5× MIC, were resuspended, diluted 1,000-fold into broth, and used to inoculate a fresh 96-well MIC plate. The MICs of a total of 20 daily passages were recorded. After passage 20, the isolate was cultured on a blood agar purity plate before determination of the final MIC.
Characterization of mutants with reduced susceptibility to REP8839.
Genomic DNA for molecular analysis was isolated using the DNeasy tissue kit (QIAGEN, Inc., Valencia, CA). A 1,067-bp fragment comprising the 5′ portion of the metS gene was amplified by PCR with high-fidelity PCR Supermix (Invitrogen, Carlsbad, CA) using primers metS1 (5′-ACATTACGAGGAGGAACAG) and metS2 (5′-GGTGTAAATACGCCATCTG). The 3′ portion of metS was amplified with primers metS3 (5′-GTCTTTGCACATGGTTGGA) and metS4 (5′-TGCTTCTCTAGCACGTGTA), yielding a 1,203-bp product. The PCR protocol for both fragments consisted of initial denaturation (5 min at 94°C); 30 cycles, with 1 cycle consisting of denaturation (1.5 min at 94°C), annealing (1 min at 55°C), and extension (1.5 min at 72°C); followed by incubation for 10 min at 72°C in a Techgene thermal cycler (Techne, Princeton, NJ). PCR products were analyzed on a 0.8% agarose-Tris-borate-EDTA gel, cloned into pCR4-TOPO (Invitrogen, Carlsbad, CA), and sequenced with T7 and T3 primers (Molecular Biology Core Facility, University of Colorado Health Sciences Center, Denver, CO). Duplicate PCR fragments were processed to minimize PCR and DNA sequencing errors. The metS DNA sequences were assembled and aligned using Vector NTI (InforMax, Bethesda, MD).
Modeling of S. aureus MetS.
Amino acid sequences for E. coli MetG apo form (Protein Data Bank identification code [PDB ID] 1QQT) and cocrystallized form with methionine (PDB ID 1F4L) were derived from the Protein Data Bank database (2). The WhatCheck (20) protein verification tool was used to assess the overall quality of the PDB files. Global pairwise amino acid sequence alignments for E. coli and S. aureus were generated with NCBI's Cn3D/MMDB sequence alignment tool (19, 48). The Needleman-Wunsch algorithm (38) and the BLOSUM62 amino acid substitution scoring matrix were used for alignments. Homology models for S. aureus used E. coli MetG apo form (PDB ID 1QQT) as the template and were created with MODELLER (35) by the satisfaction of spatial restraints (42) and the optimization of three-dimensional (3D) structure using CHARMM (Chemistry at Harvard Macromolecular Mechanics) force field energy terms. Root mean square deviation metrics were computed for the final 3D S. aureus structure relative to the E. coli template structure.
RESULTS
Biochemical activity of REP8839.
Novel diaryldiamine compounds that are potent inhibitors of S. aureus MetS have been identified (25-28). Structure-activity relationship studies led to the identification of REP8839, containing a fluorovinyl-substituted thiophene ring on the left-hand side (17). REP8839 is a highly potent (nanomolar) inhibitor of S. aureus MetS (Table 1). The 50% inhibitory concentration (IC50) was as low as the concentration of enzyme present in the assay, and the correlation between IC50 and enzyme concentration remained consistent as the enzyme concentration was varied, indicating that measurement of a true inhibitory constant is limited by the enzyme concentrations (27). It was concluded that the IC50 for REP8839 inhibition of S. aureus MetS is <1.9 nM.
TABLE 1.
Inhibition of MetS activity by REP8839
| MRS enzyme activity | IC50 (nM)a |
|---|---|
| S. aureus MetS | <1.9 |
| H. influenzae MetG | 25 |
| E. coli MetG | 307 |
| S. pneumoniae MetS2 | >500 |
| Rat liver lysateb | >500 |
The specific activity of individual enzyme preparations varied. Enzyme concentrations were adjusted to achieve charging of 15 to 25 pmol of tRNAMet in 15 min at 23°C. The enzyme concentrations were 1.5 nM for S. aureus, 3.0 nM for H. influenzae, 2.0 nM for E. coli, and 12 nM for S. pneumoniae MRS.
Rat MRS concentration was not determined; the rat liver lysate catalyzed the charging of 5 pmol of tRNAMet in 15 min at 23°C.
REP8839 also inhibited two gram-negative homologs of S. aureus MetS, H. influenzae MetG and E. coli MetG, albeit with at least 13-fold- and 160-fold-lower potency, respectively (Table 1). These studies were carried out with enzyme concentrations that yielded activity within the linear range of the assay. Due to various specific activities for the purified proteins from each organism, a somewhat different enzyme concentration was required for each preparation (Table 1). However, in contrast to the result for S. aureus MetS, the IC50 for REP8839 inhibition was significantly higher than the enzyme concentrations used in the assay for both the H. influenzae MetG (8-fold) and E. coli MetG (100-fold) enzymes, which allows determination of reliable IC50 values.
A recent study identified a second methionyl-tRNA synthetase gene, metS2, in 46% of 315 clinical isolates of S. pneumoniae (14). Interestingly, the S. pneumoniae MetS2 protein was not inhibited by potent S. aureus MetS inhibitors (14), including REP8839 (Table 1). The inhibition of a mammalian MRS activity by REP8839 was also determined, and since purified eukaryotic MRS was unavailable, we used MRS activity present in a rat liver lysate, which was not inhibited by up to 500 nM REP8839 (Table 1).
Target specificity of REP8839 in bacterial cells.
The mode of action of REP8839 in bacterial cells was evaluated to confirm the target specificity of this novel compound. First, the MetS target was overexpressed in S. aureus using a plasmid-borne copy of metS under the control of an inducible promoter, and the MICs of REP8839 and control agents are shown in Table 2. Induction of MetS expression in S. aureus RN4220(pYH4-metS) resulted in an eightfold shift in the MIC for REP8839 from 0.12 μg/ml to 1 μg/ml. Interestingly, even the uninduced S. aureus RN4220(pYH4-metS) was significantly less susceptible to REP8839 than the S. aureus RN4220(pYH4) vector control strain (MIC of 0.12 μg/ml versus MIC of 0.008 μg/ml, respectively), indicating that plasmid pYH4-metS caused some MetS expression even in the absence of inducer. The MICs for the control drugs mupirocin, novobiocin, and vancomycin remained unchanged upon MetS overexpression. These data provide good evidence that REP8839 exerts its antibacterial activity through specific inhibition of MetS in S. aureus.
TABLE 2.
MIC changes in S. aureus upon overexpression of the MetS target
| Antimicrobial agent |
S. aureus RN4220 MIC (μg/ml)
|
|||
|---|---|---|---|---|
| pYH4 vector controla
|
pYH4-metSa
|
|||
| − aTc inducer | + aTc inducer | − aTc inducer | + aTc inducer | |
| REP8839 | 0.008 | 0.008 | 0.12 | 1 |
| Mupirocin | 0.06 | 0.06 | 0.06 | 0.06 |
| Novobiocin | 0.25 | 0.25 | 0.25 | 0.25 |
| Vancomycin | 1 | 1 | 0.5 | 0.5 |
In the presence (+) or absence (−) of anhydrotetracycline (aTc) inducer.
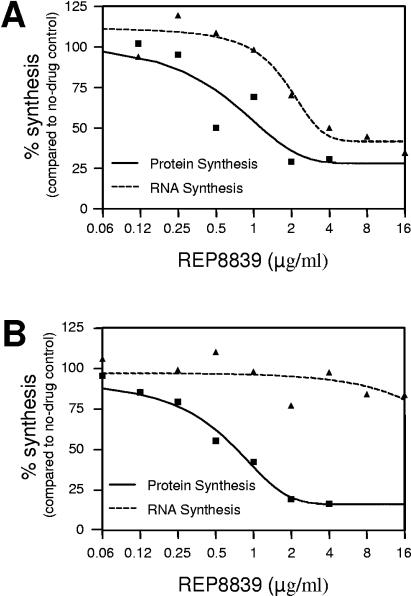
Second, macromolecular synthesis assays in S. pneumoniae in the presence of REP8839 demonstrated a dose-dependent inhibition of protein and RNA synthesis, as expected with compounds that elicit the stringent response (Fig. 1A). However, only protein synthesis was affected in a rel mutant deficient in the stringent response (Fig. 1B). These data provide direct evidence that REP8839 is a specific inhibitor of protein synthesis.
FIG. 1.
Macromolecular synthesis assay in S. pneumoniae R6 (A) and an isogenic rel mutant in which the stringent response was affected (B). Cells were treated with REP8839 for 10 min, and the incorporation of [5-3H]uridine and l-[4,5-3H]leucine was determined to measure protein synthesis and RNA synthesis.
Development of resistance to REP8839.
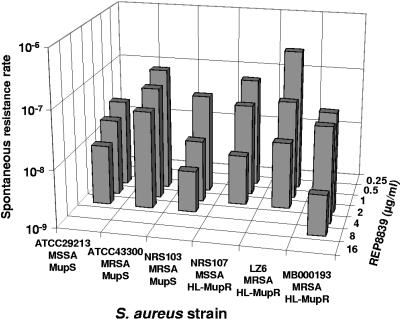
Several S. aureus strains were evaluated for their propensity to become spontaneously resistant to REP8839 when exposed to drug concentrations above the MIC. All strains tested, including MRSA and mupirocin-resistant isolates, were susceptible to REP8839 with an initial MIC range of 0.03 to 0.5 μg/ml, but most strains gave rise to first-step resistant colonies on agar containing 4 μg/ml of REP8839. S. aureus MB000193, which was initially the least susceptible strain (MIC = 0.5 μg/ml), was capable of forming colonies on agar with 8 μg/ml of REP8839. The calculated resistance rates from a population of approximately 109 cells depended on the concentration of REP8839 and were in the order of 10−7 to 10−8 after 48 h (Fig. 2). To further investigate the emergence of resistance, the MPC was determined, which is defined as the minimal drug concentration that prevents the spontaneous emergence of resistant subpopulations from 1010 cells within 5 days. For S. aureus, including methicillin-susceptible S. aureus (MSSA) and MRSA strains, the MPC was 32 μg/ml for REP8839 and greater or equal to 32 μg/ml for mupirocin (Table 3). The generation of second-step mutants was investigated by exposure of first-step mutants to even higher concentrations of REP8839. Three first-step mutants that contained different point mutations in metS and had elevated MICs of 4 to 8 μg/ml were capable of producing second-step mutants with a new MIC of 32 μg/ml at rates of 2.4 × 10−8 to 2.2 × 10−9.
FIG. 2.
Spontaneous resistance rates to REP8839 in different S. aureus strains. Approximately 109 cells were plated on agar containing REP8839 at concentrations ranging from 0.25 to 16 μg/ml, and colonies were enumerated after 48 h. MupS, mupirocin susceptible; HL-MupR, high-level mupirocin resistant.
TABLE 3.
Mutation prevention concentration for REP8839 and mupirocin in S. aureus
| S. aureus strain | MIC (μg/ml)
|
MPC (μg/ml)
|
||
|---|---|---|---|---|
| REP8839 | Mupirocin | REP8839 | Mupirocin | |
| ATCC 29213 (MSSA) | 0.03 | 0.12 | 32 | 32 |
| ATCC 43300 (MRSA) | 0.06 | 0.12 | 32 | 32 |
| LZ10 (MRSA) | 0.03 | 0.12 | 32 | >32 |
| 1079077 (MRSA) | 0.25 | 0.25 | 32 | >32 |
The propensity of staphylococci and streptococci to develop resistance to REP8839 was also evaluated in 20 serial passages of a total of 19 strains. The presence of subinhibitory concentrations of REP8839 caused the MIC for REP8839 to shift from an initial range of 0.015 to 0.06 μg/ml to a range of 0.06 to 16 μg/ml, and five strains had a MIC of 16 μg/ml after 20 passages (Table 4). There was no correlation between the decrease in susceptibility to REP8839 and the oxacillin or mupirocin resistance phenotype of the individual staphylococcal strains. In S. pyogenes the effect of passaging was less dramatic than in staphylococci, with an MIC increase from 0.12 μg/ml to 1 μg/ml. Passages with mupirocin produced a similar shift of the MIC range from 0.06 to 0.5 μg/ml to a range of 0.5 to 8 μg/ml in mupirocin-susceptible strains and from 32 μg/ml to 128 μg/ml in low-level mupirocin-resistant strains.
TABLE 4.
MIC changes in staphylococci and streptococci upon serial passages in broth containing REP8839 or mupirocin
| Straina | Phenotypeb | REP8839 MIC (μg/ml)
|
Mupirocin MIC (μg/ml)
|
||
|---|---|---|---|---|---|
| Initial | After 20 passages | Initial | After 20 passages | ||
| S. aureus ATCC 29213 | MSSA, MupS | 0.06 | 4 | 0.06 | 0.5 |
| S. aureus ATCC 43300 | MRSA, MupS | 0.06 | 1 | 0.06 | 8 |
| S. aureus LZ10 | MRSA, MupS | 0.03 | 0.5 | 0.25 | 2 |
| S. aureus NRS103 | MRSA, MupS | 0.12 | 1 | 0.25 | 1 |
| S. aureus 1079077 | MRSA, MupS | 0.25 | 16 | 0.5 | 1 |
| S. aureus 31-1334 | MRSA, LL-MupR | 0.03 | 8 | 32 | 128 |
| S. aureus 1079101 | MSSA, HL-MupR | 0.06 | 16 | 4,096 | 4,096 |
| S. aureus NRS107 | MSSA, HL-MupR | 0.015 | 0.5 | 4,096 | 4,096 |
| S. aureus LZ1 | MRSA, HL-MupR | 0.06 | 0.06 | 4,096 | 4,096 |
| S. aureus LZ6 | MRSA, HL-MupR | 0.06 | 2 | 4,096 | 4,096 |
| S. aureus 10-420 | MRSA, HL-MupR | 0.06 | 8 | 4,096 | 4,096 |
| S. aureus 87-2797 | MRSA, HL-MupR | 0.03 | 16 | 2,048 | 4,096 |
| S. aureus 25-670 | MRSA, HL-MupR | 0.06 | 8 | 2,048 | 4,096 |
| S. epidermidis NRS8 | MRSE, LL-MupR | 0.06 | 0.12 | 32 | 128 |
| S. epidermidis 936528 | MRSE, HL-MupR | 0.03 | 0.5 | 2,048 | 4,096 |
| S. epidermidis 936606 | MRSE, MupS | 0.06 | 16 | 0.12 | 2 |
| S. haemolyticus NRS116 | MRSH, MupS | 0.12 | 16 | 0.12 | 1 |
| S. pyogenes ATCC 19615 | EryS | 0.12 | 1 | 0.06 | 4 |
| S. pyogenes MB000143 | EryR | 0.12 | 1 | 0.06 | 0.5 |
Strains of S. aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, and S. pyogenes.
MupS, mupirocin susceptible; LL-MupR, low-level mupirocin resistant; HL-MupR, high-level mupirocin resistant; EryS, erythromycin susceptible; EryR, erythromycin resistant.
All of the laboratory-generated mutants with reduced susceptibility to REP8839 that were derived from mupirocin-susceptible strains remained susceptible to mupirocin (data not shown). This observation, together with the finding that all low- and high-level mupirocin-resistant strains are susceptible to REP8839 (7), indicates that there is no cross-resistance between REP8839 and mupirocin.
Molecular analysis of MetS and physiology of S. aureus with reduced susceptibility to REP8839.
The 1,974-bp metS gene that encodes the 75-kDa MetS enzyme appears to be very well conserved among different S. aureus strains. Our analysis of MetS amino acid sequences obtained from 18 S. aureus strains, including 16 clinical isolates, with an MIC range for REP8839 of 0.008 to 0.5 μg/ml revealed only a single variation. The residue at position 260 was arginine in 12 strains and lysine in 6 strains, but this was not linked to the MIC for REP8839 or to the oxacillin resistance status. A total of 89 strains obtained through spontaneous resistance to REP8839 or serial passages were characterized by DNA sequencing of the metS gene to reveal point mutations that lead to amino acid substitutions within MetS (Table 5). The majority of isolates obtained from spontaneous resistance experiments possessed an asparagine residue at position 57 instead of an isoleucine, and these I57N mutants had elevated MICs ranging from 4 to 16 μg/ml. The second type of spontaneous mutants harbored a G54S substitution in MetS, leading to MICs ranging from 16 to 32 μg/ml. These G54S mutants formed tiny colonies on agar containing REP8839 but gave rise to a subpopulation that formed large colonies on subsequent blood agar purity plates that were no longer resistant to REP8839 and were in fact true revertants in which G54 had been restored. The serial passages produced a larger variety of MetS mutations, including double and triple mutations (Table 5). I57N mutants with MICs of 8 to 16 μg/ml were still predominant, and a I57T mutant (MIC = 8 μg/ml) was also isolated. Other mutants containing a single MetS mutation leading to a MIC of 2 to 8 μg/ml were the mutants containing E52D, A61V, A77V, V108M, L213W, G223C, I238F, A247E, and V215 mutations. Nine mutants harbored two changes in MetS, and three strains contained triple mutations, which often were combinations of individual mutations found in the single mutants. All second-step mutants contained combinations of I57N or G54S with another key mutation described above, and these were the most resistant strains, with MIC of 32 μg/ml for REP8839. No mutants with MICs of >32 μg/ml were observed.
TABLE 5.
Molecular genetic characterization of laboratory-generated S. aureus mutants with decreased susceptibility to REP8839
| Isolation method | Isolate(s) | Parental strain (phenotypea) | MIC (μg/ml) | MetS mutation(s) |
|---|---|---|---|---|
| Spontaneous resistance | SSM5, others (25 isolates) | ATCC 29213 | 4-8 | 157N |
| SR5-1; SR5-2 | ATCC 43300 (MRSA) | 8 | 157N | |
| FSM 9, others (6 isolates) | 87-2797 (MRSA, HL-MupR) | 4-16 | I57N | |
| SR23-1 | LZ10 (MRSA) | 8 | I57N | |
| SR59-1, SR59-2 | 1079077 (MRSA) | 8 | I57N | |
| SR74-2 | MB000193 (MRSA) | 8 | I57N | |
| SR3, SR18, SR21 | ATCC 29213 | 16-32 | G54S | |
| FSM7 | 87-2797 (MRSA, HL-MupR) | 16 | G54S | |
| SR74-1 | MB000193 (MRSA) | 16 | G54S | |
| SR84-1, SR84-2 | MB000057 (MRSA) | 16 | G54S | |
| SR23-2 | LZ10 (MRSA) | 32 | G54S | |
| SR19, SR23 | ATCC 29213 | 0.5-1 | None | |
| FSM8 | 87-2797 (MRSA, HL-MupR) | 1 | None | |
| Serial passage | SP-1B5, SP-2D4, others (8 isolates) | ATCC 29213, ATCC 43300, others | 8-16 | I57N |
| SP-21C | 31-1334 (MRSA, LL-MupR) | 8 | I57T | |
| SP-27H | 10-420 (MRSA, HL-MupR) | 4 | E52D | |
| SP-27D | 14-354 (MRSA, LL-MupR) | 2 | A61V | |
| SP-21A | ATCC 29213 | 4 | A77V | |
| SP-25G | LZ6 (MRSA, HL-MupR) | 8 | V108M | |
| SP-2C4 | ATCC 43300 (MRSA) | 2 | L213W | |
| SP-28B | 25-670 (MRSA, HL-MupR) | 4 | V215A | |
| SP-4A2 | 87-2797 (MRSA, HL-MupR) | 8 | G223C | |
| SP-11A3 | 31-1334 (MRSA, LL-MupR) | 8 | 1238F | |
| SP-1A2 | ATCC 29213 | 4 | A247E | |
| SP-9B5 | ATCC 29213 | 4 | I57N V296F | |
| SP-2B5 | ATCC 43300 (MRSA) | 32 | I57N R100S | |
| SP-4B5 | 87-2797 (MRSA, HL-MupR) | 32 | I57N V242F | |
| SP-25C | 31-1334 (MRSA, LL-MupR) | 2 | T50A V242I | |
| SP-26C | 1079101 (HL-MupR) | 32 | G54A A64P | |
| SP-25F | LZ1 (MRSA, HL-MupR) | 16 | A61T A64S | |
| SP-22B | 25-670 (MRSA, HL-MupR) | 8 | A61T V108L | |
| SP-21G | LZ6 (MRSA, HL-MupR) | 2 | I94N V215A | |
| SP-21H | 10-420 (MRSA, HL-MupR) | 8 | V215I V242F | |
| SP-22C | 1079101 (HL-MupR) | 16 | L19F Q58L A64P | |
| SP-25D | 14-354 (MRSA, LL-MupR) | 8 | A61T E98G M269I | |
| SP-26B | 25-670 (MRSA, HL-MupR) | 4 | P230T A247E L257P | |
| SP-21B | ATCC 43300 (MRSA) | 2 | None | |
| SP-3A5 | 31-1334 (MRSA, LL-MupR) | 2 | None | |
| SP-4D5 | 87-2797 (MRSA, HL-MupR) | 0.25 | None | |
| Second-step mutations | SSM 1-01, SSM 1-02, SSM 1-03 | ATCC 29213 | 32 | I57N A247E |
| SSM 6-01 | ATCC 29213 | 32 | I57N G54S | |
| SSM 6-02, 6-03 | ATCC 29213 | 32 | I57N I238F | |
| SSM 14-01, 14-02 | LZ6 (MRSA, HL-MupR) | 32 | G54S V108M |
HL-MupR, high-level mupirocin resistant; LL-MupR, low-level mupirocin resistant.
The characterization of MetS mutants with decreased susceptibility to REP8839 provides further evidence that REP8839 is a specific inhibitor of MetS in S. aureus. Only a few spontaneous mutants or serial passage isolates did not have any alterations in MetS (e.g., SR19, SR23, FSM8, SP-21B, SP-3A, and SP-4D), however, their susceptibilities to REP8839 were only slightly decreased (MIC = 0.25 to 2 μg/ml).
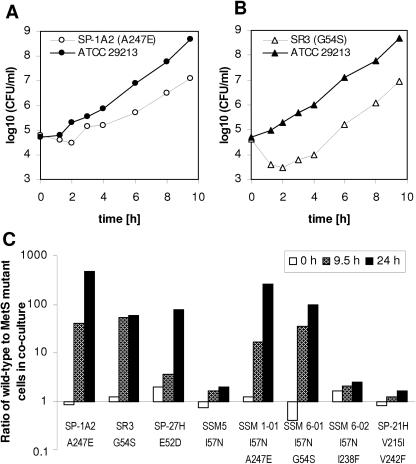
The effects of MetS mutations on fitness were assessed in competition cocultures of S. aureus MetS mutants with wild-type cells. The most dramatic growth reduction was detected in strain SP-1A2 containing an A247E mutation (Fig. 3A) and in strain SR3 containing G54S (Fig. 3B). The cost-of-fitness index, which we defined as the ratio of wild-type cells to MetS mutant cells after exponential growth, was between 50 and 500 for all mutants that harbored A247E or G54S substitutions, meaning that they were unable to compete with wild-type cells (Fig. 3C). SP-27H cells containing an E52D substitution in MetS were outnumbered by wild-type cells 4-fold at 9.5 h, but 78-fold at 24 h (Fig. 3C), suggesting that competition continued during stationary phase in an equilibrium between dying and growing cells. Other mutants, including SSM5 (I57N), SSM 6-02 (I57N I238F), and SP-21H (V215I V242F) remained relatively fit, with cost-of-fitness indices of 1.6 to 2.5 (Fig. 3C).
FIG. 3.
Fitness burden due to MetS mutations. Wild-type cells were grown in competition cocultures with SP-1A2 cells (MetS with A247E) (A) or with SR3 cells (MetS with G54S) (B). Diluted samples were plated on blood agar to enumerate total cells or on selective agar containing REP8839 to enumerate MetS mutant cells. Increased ratios of wild-type cells to MetS mutant cells in the mixed cultures illustrate the varying cost of fitness (C).
Comparison of S. aureus MetS and E. coli MetG and molecular modeling.
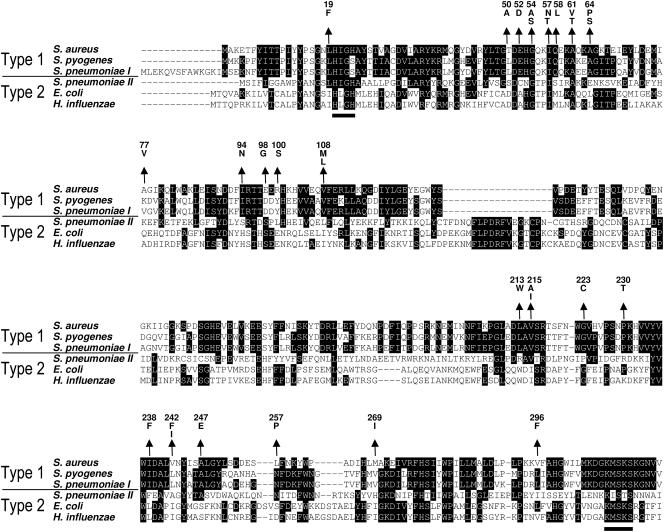
All mutations leading to a higher MIC for REP8839 were located within the amino-terminal half of the MetS enzyme, which is in agreement with the fact that the carboxy-terminal portion contains the tRNA binding domain and thus seems unlikely to be affected by binding of REP8839 (12). An alignment of type 1 and type 2 MRS amino acid sequences of representative species is depicted in Fig. 4 with the locations of all characterized mutations. The region from amino acid residues 50 to 64 was most frequently affected in MetS mutants and contains G54, I57, and A61, all of which are conserved within the six bacterial strains, and A64, which is conserved among strains harboring type 1 MRS. Interestingly, several residues of altered S. aureus MetS had changed to the corresponding amino acids found at these positions in type 2 MRS, for which REP8839 has a much lower affinity, such as 213W, 215I, 238F, and 269I. None of the mutations were located within the well-conserved ATP-binding Rossman fold that contains the motifs H(M/I)GH and KMSKS, which characterize the class I tRNA synthetases.
FIG. 4.
Alignment of MRS type 1 and type 2 amino acid sequences. Conserved residues are indicated by white letters on black background, and the H(M/I)GH and KMSKS motifs of the ATP-binding Rossman fold that are typically found in all class I tRNA synthetases are underlined. Arrows indicate the positions and point to amino acid changes in S. aureus mutants with decreased susceptibility to REP8839. Gaps introduced to maximize alignment are indicated by dashes.
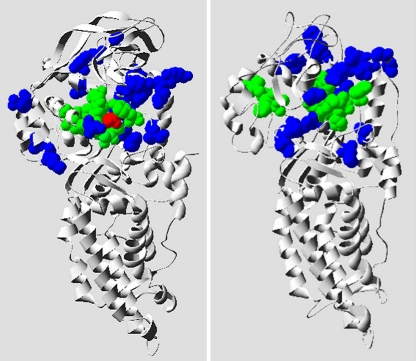
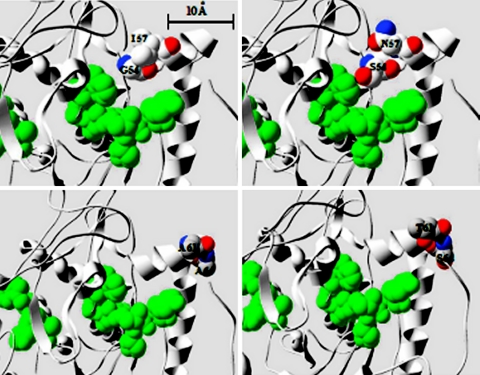
A structural model of S. aureus MetS was generated on the basis of the known E. coli MetG apo form (PDB ID 1QQT) and the amino acid sequence alignment that showed 38% similarity. The accuracy of homology models with 40% sequence similarity is typically as good as nuclear magnetic resonance-derived structures (5). E. coli MetG and S. aureus MetS appear to possess a considerably conserved 3D structure, as depicted in Fig. 5. All 23 amino acid substitutions found within mutant MetS proteins were clustered around the active site where the ligand methionine is bound. The amino acid substitutions in mutant MetS proteins from strain SSM 6-01 (I57N G54S) and SP-25F (A61T A64S) are located in close proximity to active-site residues (Fig. 6). Their larger size and their hydrophilic nature may impair MetS function considerably and may cause the observed fitness burden of strain SSM 6-01.
FIG. 5.
Model of the S. aureus MetS apo form. The E. coli MetG cocrystallized form (PDB ID 1F4L) is shown in the left panel, with the methionine substrate highlighted in red. The right panel depicts a 3D model of the S. aureus MetS apo form that was generated on the basis of the E. coli MetG apo form as a template (PDB ID 1QQT). The 10 active-site residues within MRS are shown in green, and the positions of the residues affected in S. aureus MetS mutants with decreased susceptibility to REP8839 are shown in blue.
FIG. 6.
Comparison of modeled active sites of S. aureus wild-type and mutant MetS. The key residues (colored) proximal to the active-site residues that outline the methionine/ATP binding pocket (green) caused the largest shift in MIC for REP8839 when altered. (Top) Wild-type MetS (I57 G54) and mutant MetS (N57 S54). (Bottom) Wild-type MetS (A61 A64) and mutant MetS (T61 S64).
DISCUSSION
Aminoacyl-tRNA synthetases represent novel molecular targets for the development of new classes of antibacterial agents. Pathogen-specific inhibition of this family of universal and essential targets has been validated for mupirocin, which selectively inhibits the bacterial isoleucyl-tRNA synthetase without affecting the mammalian counterpart (22). Many years of using topical formulations containing 2% mupirocin to eradicate S. aureus from the anterior nares and to prevent infections in carriers have resulted in a substantial increase in resistance rates. It is believed that low drug concentrations in the pharynx posttreatment may increase the risk of emergence of resistance, and this has been proposed as a mechanism for the development of mupirocin-resistant strains (49). A novel agent, REP8839, is being developed as a topical agent targeting the methionyl-tRNA synthetase. REP8839 is fully synthetic, and thus the lack of preexisting resistance is not surprising. However, natural resistance to REP8839 occurs in S. pneumoniae, which harbors a second gene (metS2) that may have been acquired through horizontal transfer (3). The distribution of metS2 appears to be limited to S. pneumoniae, Bacillus anthracis, Bacillus cereus, and Clostridium perfringens (3). In fact, all strains of S. pyogenes (n > 50) and S. aureus (n > 130) examined so far contained only metS1, not metS2, and were susceptible to REP8839 (data not shown).
The binding of REP8839 to S. aureus MetS was so potent that determinations of an IC50 essentially resulted in enzyme titration. This was also observed previously for other similarly active MRS inhibitors (26, 27) and is reminiscent of the effects seen for the inhibition of IRS by mupirocin, which is a two-step process in which the initial enzyme-inhibitor complex undergoes an isomerization to form a tightened enzyme-inhibitor complex (40). The dissociation constant for mupirocin is approximately 20 pM, with a half-life of 140 min (40).
The mode of action of REP8839 was examined in whole-cell macromolecular synthesis assays. Inhibition of a tRNA synthetase essentially mimics starvation for amino acids by lowering the ratio of charged to uncharged tRNA within the cell. All known inhibitors of tRNA synthetases with whole-cell activity induce a stringent response, leading to a rapid decrease in the incorporation of radiolabeled uridine and (p)ppGpp levels. In contrast, “relaxed” strains with mutations in the (p)ppGpp synthetase gene do not exhibit a decrease in RNA synthesis. REP8839 inhibited both RNA and protein synthesis in S. pneumoniae R6, while only protein synthesis was reduced in an isogenic rel mutant strain, providing evidence for tRNA synthetase inhibition by REP8839.
Target specificity of REP8839 was also demonstrated through resistance studies. REP8839 was essentially equal to mupirocin regarding the MPC and was superior to mupirocin regarding the level of resistance in stable spontaneous mutants. The highest MIC for REP8839 was 32 μg/ml, which is several orders of magnitude below the drug concentration of 2% (20,000 μg/ml) in a typical topical agent. However, the local drug concentration at the site of infection may vary, and it is a known fact that exposure to topical agents containing 2% mupirocin or fusidic acid can select for low-level resistance. Reduced susceptibility to REP8839 was associated with varying cost of fitness. In a report on mupirocin resistance, first-step mutations (V588F and V631F) in IRS were generally not associated with fitness costs, but second-step mutants were unfit and produced compensatory mutations to restore fitness (24).
Our collection of MetS mutants may prove useful for gaining further insight into the drug-target interactions. Attempts to obtain crystals of the S. aureus MetS enzyme have failed, but crystallographic studies on E. coli MetG have been more successful, both in native form and as a complex with methionine (37, 45). The hydrophobic binding pocket consists of 10 amino acids surrounding the l-methionine, whose amine group is hydrogen bonded to the carboxyl group of Asp52 and the carbonyl oxygen atom of Leu13 (45). In a recent study, two analogs of REP8839 were docked into the binding pocket of E. coli MetG using the comparative molecular field analysis method and were found to form hydrogen bonds to Asp296 and to a water molecule (30). Although the overall identity between S. aureus MetS and E. coli MetG is only 26%, the two proteins are similar in length (658 versus 677 residues, respectively) and share key residues implicated in substrate binding. Interestingly, G54 and I57 that were identified in our study as the most frequent sites of substitutions leading to reduced REP8839 susceptibility are conserved in E. coli and S. aureus and are very near the Asp52 residue that forms a hydrogen bond to methionine in E. coli MetG. Moreover, many MetS mutations occur in the region from amino acids 213 to 296, which is located near the binding pocket and centered around the Val252 residue that is conformationally constrained in the E. coli enzyme upon inhibitor binding.
In the energy minimization model for one of the representatives of this class (E. coli MetG), the REP8839 binding site overlaps the known binding site of methionine (30). Our model of the S. aureus MetS active site was created using the known crystal structure of the E. coli MetG apo form (PDB ID 1QQT) as the template. Strikingly, all of the amino acid substitutions in our collection of S. aureus MetS mutants with decreased susceptibility to REP8839 are located around the active site. The amino acid changes I57N G54S (strain SSM 6-01, MIC = 32 μg/ml) and A61T A64S (strain SP-25F, MIC = 16 μg/ml) clearly extend into the active site. Furthermore, the substitution of the small nonpolar side chains found in wild-type MetS with the bulkier and polar side chains in these mutants likely affects the hydrophobic pocket, leading to lower affinity for REP8839. Studies are under way to investigate the precise roles of individual mutant protein residues in substrate binding, inhibitor binding, and enzyme kinetics.
Acknowledgments
We thank GlaxoSmithKline for providing bacterial strains, plasmids, and purified proteins used in this study. We thank Richard Casey and Eric Weber from RMC Biosciences (Fort Collins, CO) for modeling the S. aureus MetS apo form and active site.
REFERENCES
- 1.Antonio, M., N. McFerran, and M. J. Pallen. 2002. Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 46:438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman, H. M., J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov, and P. E. Bourne. 2000. The Protein Data Bank. Nucleic Acids Res. 28:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, J. R., D. Gentry, J. A. Becker, K. Ingraham, D. J. Holmes, and M. J. Stanhope. 2003. Horizontal transfer of drug-resistant aminoacyl-transfer-RNA synthetases of anthrax and Gram-positive pathogens. EMBO Rep. 4:692-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caelli, M., J. Porteous, C. F. Carson, R. Heller, and T. V. Riley. 2000. Tea tree oil as an alternative topical decolonization agent for methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 46:236-237. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarty, S., L. Wang, and R. Sanchez. 2005. Accuracy of structure-derived properties in simple comparative models of protein structures. Nucleic Acids Res. 33:244-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing, 15th informational supplement. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 7.Critchley, I. A., C. L. Young, K. C. Stone, U. A. Ochsner, J. Guiles, T. Tarasow, and N. Janjic. 2005. Antibacterial activity of REP8839, a new antibiotic for topical use. Antimicrob. Agents Chemother. 49:4247-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler, R. R., and P. Wilson. 2004. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 61:71-74. [DOI] [PubMed] [Google Scholar]
- 9.Davis, K. A., J. J. Stewart, H. K. Crouch, C. E. Florez, and D. R. Hospenthal. 2004. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin. Infect. Dis. 39:776-782. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande, L. M., A. M. Fix, M. A. Pfaller, and R. N. Jones. 2002. Emerging elevated mupirocin resistance rates among staphylococcal isolates in the SENTRY Antimicrobial Surveillance Program (2000): correlations of results from disk diffusion, Etest and reference dilution methods. Diagn. Microbiol. Infect. Dis. 42:283-290. [DOI] [PubMed] [Google Scholar]
- 11.Enright, M. C. 2003. The evolution of a resistant pathogen—the case of MRSA. Curr. Opin. Pharmacol. 3:474-479. [DOI] [PubMed] [Google Scholar]
- 12.Eriani, G., M. Delarue, O. Poch, J. Gangloff, and D. Moras. 1990. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 347:203-206. [DOI] [PubMed] [Google Scholar]
- 13.Farmer, T. H., J. Gilbart, and S. W. Elson. 1992. Biochemical basis of mupirocin resistance in strains of Staphylococcus aureus. J. Antimicrob. Chemother. 30:587-596. [DOI] [PubMed] [Google Scholar]
- 14.Gentry, D. R., K. A. Ingraham, M. J. Stanhope, S. Rittenhouse, R. L. Jarvest, P. J. O'Hanlon, J. R. Brown, and D. J. Holmes. 2003. Variable sensitivity to bacterial methionyl-tRNA synthetase inhibitors reveals subpopulations of Streptococcus pneumoniae with two distinct methionyl-tRNA synthetase genes. Antimicrob. Agents Chemother. 47:1784-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbart, J., C. R. Perry, and B. Slocombe. 1993. High-level mupirocin resistance in Staphylococcus aureus: evidence for two distinct isoleucyl-tRNA synthetases. Antimicrob. Agents Chemother. 37:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood, R. C., and D. R. Gentry. 2002. Confirmation of the antibacterial mode of action of SB-219383, a novel tyrosyl tRNA synthetase inhibitor from a Micromonospora sp. J. Antibiot. (Tokyo) 55:423-426. [DOI] [PubMed] [Google Scholar]
- 17.Guiles, J., T. Tarasow, I. A. Critchley, K. Stone, C. Young, U. Ochsner, and N. Janjic. 2004. Novel thiophene-containing inhibitors of bacterial methionyl tRNA synthetase: improved activity resulting from fluorovinylthiophene replacement of arylbromides, abstr F-727, p. 204-205. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 18.Hodgson, J. E., S. P. Curnock, K. G. Dyke, R. Morris, D. R. Sylvester, and M. S. Gross. 1994. Molecular characterization of the gene encoding high-level mupirocin resistance in Staphylococcus aureus J2870. Antimicrob. Agents Chemother. 38:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogue, C. W. 1997. Cn3D: a new generation of three-dimensional molecular structure viewer. Trends Biochem. Sci. 22:314-316. [DOI] [PubMed] [Google Scholar]
- 20.Hooft, R. W., G. Vriend, C. Sander, and E. E. Abola. 1996. Errors in protein structures. Nature 381:272. [DOI] [PubMed] [Google Scholar]
- 21.Hudson, I. R. 1994. The efficacy of intranasal mupirocin in the prevention of staphylococcal infections: a review of recent experience. J. Hosp. Infect. 27:81-98. [DOI] [PubMed] [Google Scholar]
- 22.Hughes, J., and G. Mellows. 1980. Interaction of pseudomonic acid A with Escherichia coli B isoleucyl-tRNA synthetase. Biochem. J. 191:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurdle, J. G., A. J. O'Neill, and I. Chopra. 2004. Anti-staphylococcal activity of indolmycin, a potential topical agent for control of staphylococcal infections. J. Antimicrob. Chemother. 54:549-552. [DOI] [PubMed] [Google Scholar]
- 24.Hurdle, J. G., A. J. O'Neill, E. Ingham, C. Fishwick, and I. Chopra. 2004. Analysis of mupirocin resistance and fitness in Staphylococcus aureus by molecular genetic and structural modeling techniques. Antimicrob. Agents Chemother. 48:4366-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvest, R. L., S. A. Armstrong, J. M. Berge, P. Brown, J. S. Elder, M. J. Brown, R. C. Copley, A. K. Forrest, D. W. Hamprecht, P. J. O'Hanlon, D. J. Mitchell, S. Rittenhouse, and D. R. Witty. 2004. Definition of the heterocyclic pharmacophore of bacterial methionyl tRNA synthetase inhibitors: potent antibacterially active non-quinolone analogues. Bioorg. Med. Chem. Lett. 14:3937-3941. [DOI] [PubMed] [Google Scholar]
- 26.Jarvest, R. L., J. M. Berge, V. Berry, H. F. Boyd, M. J. Brown, J. S. Elder, A. K. Forrest, A. P. Fosberry, D. R. Gentry, M. J. Hibbs, D. D. Jaworski, P. J. O'Hanlon, A. J. Pope, S. Rittenhouse, R. J. Sheppard, C. Slater-Radosti, and A. Worby. 2002. Nanomolar inhibitors of Staphylococcus aureus methionyl tRNA synthetase with potent antibacterial activity against gram-positive pathogens. J. Med. Chem. 45:1959-1962. [DOI] [PubMed] [Google Scholar]
- 27.Jarvest, R. L., J. M. Berge, M. J. Brown, P. Brown, J. S. Elder, A. K. Forrest, C. S. Houge-Frydrych, P. J. O'Hanlon, D. J. McNair, S. Rittenhouse, and R. J. Sheppard. 2003. Optimisation of aryl substitution leading to potent methionyl tRNA synthetase inhibitors with excellent gram-positive antibacterial activity. Bioorg. Med. Chem. Lett. 13:665-668. [DOI] [PubMed] [Google Scholar]
- 28.Jarvest, R. L., J. M. Berge, P. Brown, C. S. Houge-Frydrych, P. J. O'Hanlon, D. J. McNair, A. J. Pope, and S. Rittenhouse. 2003. Conformational restriction of methionyl tRNA synthetase inhibitors leading to analogues with potent inhibition and excellent gram-positive antibacterial activity. Bioorg. Med. Chem. Lett. 13:1265-1268. [DOI] [PubMed] [Google Scholar]
- 29.Jones, M. E., D. C. Draghi, C. Thornsberry, J. A. Karlowsky, D. F. Sahm, and R. P. Wenzel. 29. July 2004, posting date. Emerging resistance among bacterial pathogens in the intensive care unit—a European and North American Surveillance study (2000-2002). Ann. Clin. Microbiol. Antimicrob. 3:14. [Online.] http://www.ann-clinmicrob.com/content/3/1/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, S. Y., and J. Lee. 2003. 3-D-QSAR study and molecular docking of methionyl-tRNA synthetase inhibitors. Bioorg. Med. Chem. 11:5325-5331. [DOI] [PubMed] [Google Scholar]
- 31.Kokai-Kun, J. F., S. M. Walsh, T. Chanturiya, and J. J. Mond. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob. Agents Chemother. 47:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laupland, K. B., and J. M. Conly. 2003. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin. Infect. Dis. 37:933-938. [DOI] [PubMed] [Google Scholar]
- 33.Long, T. E. 2003. Recent progress toward the clinical development of new anti-MRSA antibiotics. IDrugs 6:351-359. [PubMed] [Google Scholar]
- 34.Macarron, R., L. Mensah, C. Cid, C. Carranza, N. Benson, A. J. Pope, and E. Diez. 2000. A homogeneous method to measure aminoacyl-tRNA synthetase aminoacylation activity using scintillation proximity assay technology. Anal. Biochem. 284:183-190. [DOI] [PubMed] [Google Scholar]
- 35.Marti-Renom, M. A., A. C. Stuart, A. Fiser, R. Sanchez, F. Melo, and A. Sali. 2000. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 29:291-325. [DOI] [PubMed] [Google Scholar]
- 36.Mason, B. W., and A. J. Howard. 2004. Fusidic acid resistance in community isolates of methicillin susceptible Staphylococcus aureus and the use of topical fusidic acid: a retrospective case-control study. Int. J. Antimicrob. Agents 23:300-303. [DOI] [PubMed] [Google Scholar]
- 37.Mechulam, Y., E. Schmitt, L. Maveyraud, C. Zelwer, O. Nureki, S. Yokoyama, M. Konno, and S. Blanquet. 1999. Crystal structure of Escherichia coli methionyl-tRNA synthetase highlights species-specific features. J. Mol. Biol. 294:1287-1297. [DOI] [PubMed] [Google Scholar]
- 38.Needleman, S. B., and C. D. Wunsch. 1970. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 48:443-453. [DOI] [PubMed] [Google Scholar]
- 39.Pappa, K. A. 1990. The clinical development of mupirocin. J. Am. Acad. Dermatol. 22:873-879. [DOI] [PubMed] [Google Scholar]
- 40.Pope, A. J., K. J. Moore, M. McVey, L. Mensah, N. Benson, N. Osbourne, N. Broom, M. J. Brown, and P. O'Hanlon. 1998. Characterization of isoleucyl-tRNA synthetase from Staphylococcus aureus. II. Mechanism of inhibition by reaction intermediate and pseudomonic acid analogues studied using transient and steady-state kinetics. J. Biol. Chem. 273:31691-31701. [DOI] [PubMed] [Google Scholar]
- 41.Rayner, D. 2003. MRSA: an infection control overview. Nurs. Stand. 17:47-53. [DOI] [PubMed] [Google Scholar]
- 42.Sali, A., and T. L. Blundell. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234:779-815. [DOI] [PubMed] [Google Scholar]
- 43.Schuenck, R. P., P. Dadalti, M. G. Silva, L. S. Fonseca, and K. R. Santos. 2004. Oxacillin- and mupirocin-resistant Staphylococcus aureus: in vitro activity of silver sulphadiazine and cerium nitrate in hospital strains. J. Chemother. 16:453-458. [DOI] [PubMed] [Google Scholar]
- 44.Semret, M., and M. A. Miller. 2001. Topical mupirocin for eradication of MRSA colonization with mupirocin-resistant strains. Infect. Control Hosp. Epidemiol. 22:578-580. [DOI] [PubMed] [Google Scholar]
- 45.Serre, L., G. Verdon, T. Choinowski, N. Hervouet, J. L. Risler, and C. Zelwer. 2001. How methionyl-tRNA synthetase creates its amino acid recognition pocket upon L-methionine binding. J. Mol. Biol. 306:863-876. [DOI] [PubMed] [Google Scholar]
- 46.Vandenesch, F., and J. Etienne. 1 November 2004, posting date. How to prevent transmission of MRSA in the open community? Euro. Surveill., vol. 9. [Online.] http://www.eurosurveillance.org/em/v09n11/0911-221.asp. [DOI] [PubMed]
- 47.Walker, E. S., J. E. Vasquez, R. Dula, H. Bullock, and F. A. Sarubbi. 2003. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus: does mupirocin remain effective? Infect. Control Hosp. Epidemiol. 24:342-346. [DOI] [PubMed] [Google Scholar]
- 48.Wang, Y., J. B. Anderson, J. Chen, L. Y. Geer, S. He, D. I. Hurwitz, C. A. Liebert, T. Madej, G. H. Marchler, A. Marchler-Bauer, A. R. Panchenko, B. A. Shoemaker, J. S. Song, P. A. Thiessen, R. A. Yamashita, and S. H. Bryant. 2002. MMDB: Entrez's 3D-structure database. Nucleic Acids Res. 30:249-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe, H., H. Masaki, N. Asoh, K. Watanabe, K. Oishi, S. Kobayashi, A. Sato, R. Sugita, and T. Nagatake. 2001. Low concentrations of mupirocin in the pharynx following intranasal application may contribute to mupirocin resistance in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 39:3775-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White, C. 2004. MRSA infections rose by 5% between 2003 and 2004. BMJ 329:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilcox, M. H., J. Hall, A. B. Gill, W. N. Fawley, P. Parnell, and P. Verity. 2004. Effectiveness of topical chlorhexidine powder as an alternative to hexachlorophane for the control of Staphylococcus aureus in neonates. J. Hosp. Infect. 56:156-159. [DOI] [PubMed] [Google Scholar]