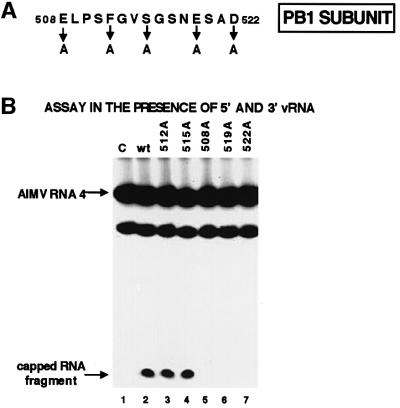
Fig. 4. Identification of the polymerase active site that is required for the catalytic function of the endonuclease. (A) Amino acid sequence of the region in the PB1 protein subunit that cross-links to the capped RNA oligonucleotide containing thio U close to the site of endo nucleolytic cleavage. The positions of the five amino acids that were individually replaced with an alanine are denoted. (B) Cap-dependent endonuclease activities of wild-type and mutant polymerases. After binding 5′ vRNA and 3′ vRNA to the polymerase complexes, wild-type (lane 2) or mutant polymerases (lanes 3–7) were incubated with full-length AlMV RNA 4 containing an m7G32pppGm 5′ end. The RNA products were separated on a denaturing 20% gel. The positions of full-length ALMV RNA 4 and of the specific 13-nt-long cleavage product are indicated by arrows. The species migrating faster than intact ALMV RNA 4 is a non-specific breakdown product. Lane 1 (C), full-length AlMV RNA 4 incubated in the absence of a polymerase complex.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.