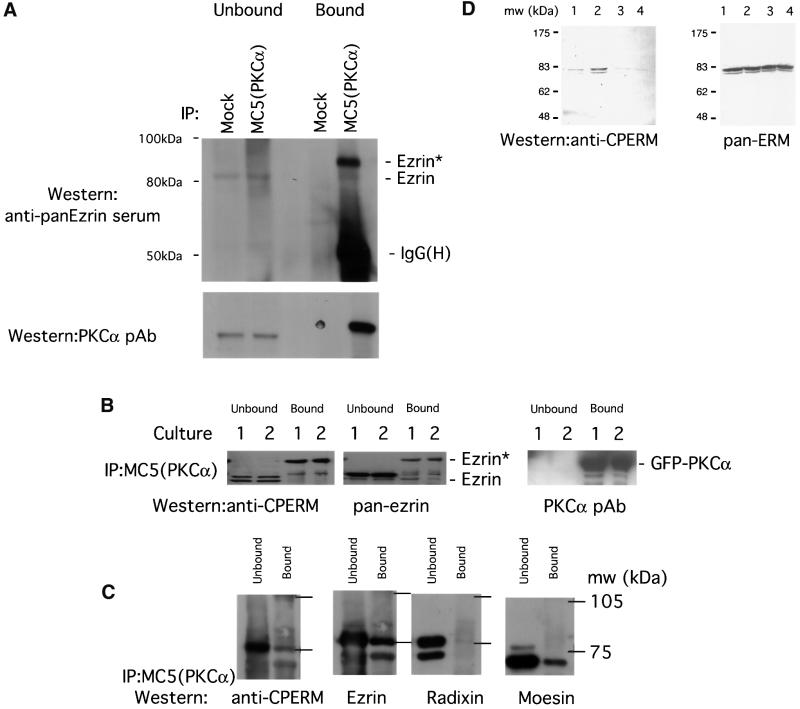
Fig. 5. Association of endogenous ERM proteins with PKCα in vivo correlated with an increase in C-terminal threonine phosphorylation. (A) MDA-MB-231 cells stimulated with PDBu. Co-precipitation of endogenous PKCα (immunoprecipitated with an anti-PKCα mAb MC5) with endogenous ezrin detected by an anti-ezrin rabbit serum. All the bound proteins on the protein G beads (bound) and 1/30 of the unbound proteins left in the cell extract supernatant after the first centrifugation post-precipitation (unbound) were loaded. Ezrin* is a subset of ezrin that exhibits a slower electrophoretic mobility and is only detected in the PKCα-bound ezrin pool. The blot was stripped and reprobed with an anti-PKCα pAb. Mock, control immunoprecipitation in which the precipitating antibody was omitted; IgG(H), immunoglobulin heavy chain. Approximate positions of the molecular weight markers are shown. (B) GFP–PKCα-2C4 cells stimulated with PDBu. Co-precipitation of GFP–PKCα with endogenous ezrin. All the bound proteins on the protein G beads (bound) and 1/100 of the unbound proteins left in the cell extract supernatant after the first centrifugation post-precipitation (unbound) were loaded. The blot containing MC5 immunoprecipitates, derived from two independent experiments (cultures 1 and 2), was detected first with a polyclonal rabbit anti-CPERM IgG, stripped and reprobed with an anti-ezrin rabbit serum, then stripped and reprobed again with an anti-PKCα pAb. (C) Relative proportion of total ERM bound to GFP–PKCα in unstimulated GFP–PKCα-2C4 cells. MC5 immuno precipitates from three untreated cultures were pooled and one-third of the pooled bound proteins on the protein G beads (bound) were loaded. Similarly, 1/300 of the pooled unbound proteins left in the cell extract supernatant after the first centrifugation post-precipitation (unbound) were loaded. The blot was cut into three strips and stained with (i) an affinity-purified polyclonal rabbit anti-CPERM IgG and crude sera raised against radixin (ii) and moesin (iii), respectively. The anti-CPERM blot (i) was then stripped and reprobed with an affinity-purified anti-ezrin IgG that has been cross-adsorbed for moesin and radixin. (D) In total LLC-PK1 cell lysates, immunoreactivity of ERM proteins with the affinity-purified polyclonal rabbit anti-CPERM IgG was abolished by pre-treatment of cells with staurosporine (a broad spectrum protein kinase inhibitor) before cell lysis, and enhanced by calyculin A, a protein serine/threonine phosphatase 1 (PP1)/PP2A inhibitor. ERM, immunoblot developed with a mixture of antibodies specific for ERM (Gautreau et al., 2000). Lane 1, untreated; lane 2, treated with calyculin A (100 nM, 10 min); lanes 3 and 4, treated with 1 and 5 µM staurosporine, respectively, for 10 min. Approximate positions of the molecular weight markers are shown.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.