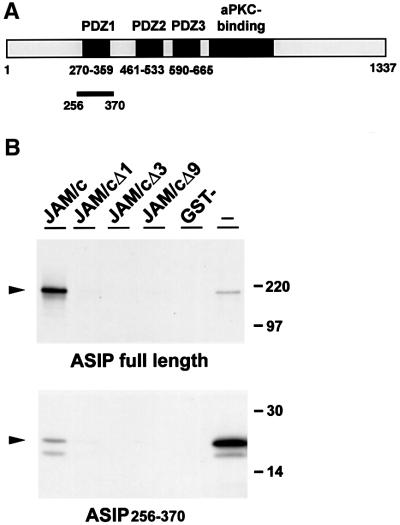
Fig. 1. ASIP interacts directly with JAM. (A) Schematic organization of ASIP. The PDZ domains are drawn as black boxes; the aPKC- interacting domain is drawn in grey. The black bar indicates the ASIP fragment isolated from the yeast two-hybrid library. (B) Full-length ASIP and the ASIP fragment isolated from the yeast two-hybrid library (ASIP256–370) were generated in vitro in the presence of [35S]methionine and incubated with immobilized GST fusion proteins containing the complete cytoplasmic tail of JAM (JAM/c), the cytoplasmic tail lacking one (JAM/cΔ1), three (JAM/cΔ3) or nine (JAM/cΔ9) C-terminal amino acid residues, or with GST alone (GST-). Both full-length ASIP and the isolated fragment comprising the first PDZ domain of ASIP interact with JAM in a PDZ-domain-dependent manner. The association of full-length ASIP is much stronger than that of the short ASIP fragment. In lanes marked with (–), aliquots of the translation reaction were loaded to indicate the Mr of the generated protein. Both constructs were generated in vitro with comparable efficiencies (data not shown); the different intensities of the signals in the (–) lanes are due to a longer exposure of the gel. The second band at a lower molecular weight represents a degradation product of in vitro generated ASIP.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.