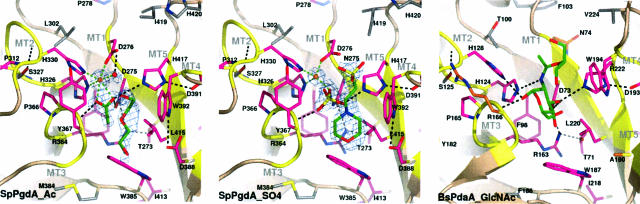
Fig. 2.
Details of the SpPgdA active site. Close-up of the active sites of: the native SpPgdA structure in complex with the acetate product and PEG200 (SpPGDA_Ac), the SpPgdA D275N mutant in complex with sulfate and Mes (SpPGDA_SO4), and the previously determined complex of B. subtilis PdaA in complex with GlcNAc and a glycerol molecule (PDAA_GlcNAc). The five CE-4 sequence motifs (MT1–5) are shown in yellow. Side chains lining the active site cleft are shown as sticks. Residues conserved in all CE-4 esterases are magenta. Water molecules (spheres) and ligands (green sticks) are also shown. Unbiased Fo - Fc, φcalc maps are shown at 2.25 σ (Mes in SpPGDA_SO4) and 12 σ (Zn in SpPGDA_Ac/SO4). Hydrogen bonds are shown as dashed lines in black and zinc–ligand interactions, as green dashed lines.