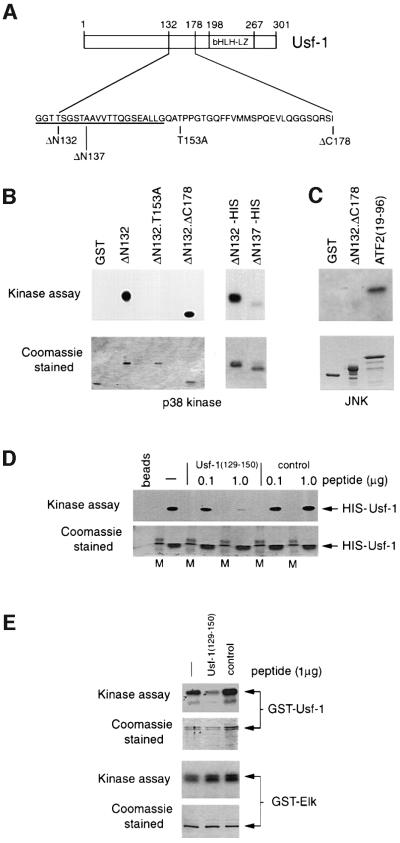
Fig. 4. Requirements within Usf-1 for stress-induced phosphorylation in vitro. (A) Schematic of Usf-1 proteins used in the in vitro kinase assays. (B) Usf-1 is phosphorylated by p38α in vitro. In vitro kinase assays were performed using either purified GST–Usf-1 fusion protein or His6-tagged Usf-1 proteins in the presence of recombinant activated p38α kinase [γ-32P]ATP. Kinase assays using the indicated Usf-1 derivatives are shown in the upper panel after SDS–PAGE and autoradiography. The lower panel shows the same gel stained with Coomassie Blue indicating that equivalent amounts of protein were used in each assay. (C) JNK does not efficiently phosphorylate Usf-1. The indicated GST fusion proteins were incubated with purified active JNK and [γ-32P]ATP. Efficient phosphorylation of ATF2 was observed but not of either GST alone or the GST-Usf-1 fusion protein. (D) A competitor peptide can inhibit the phosphorylation of His-tagged Usf-1 by p38α in vitro. Active recombinant p38α kinase was pre-incubated with either Usf-1 competitor peptide corresponding to Usf-1 amino acids 129–150, or a control peptide (0.1–1 µg) derived from the Pho4 activation domain, for 30 min prior to the kinase reaction in the presence of His-tagged Usf-1 recombinant protein as indicated. M indicates a molecular weight marker. (E) The Usf-1 peptide inhibits phosphorylation of Usf-1 but not Elk by p38. Kinase assays were performed as in (D) using the indicated GST fusion proteins in the presence or absence of the Usf-1 peptide or the control Pho4 peptide.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.