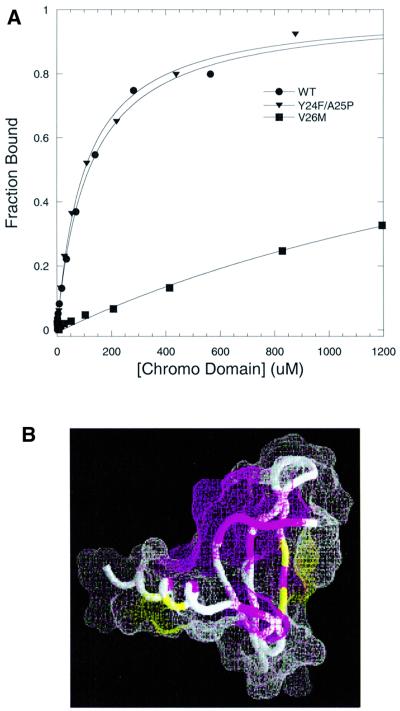
Fig. 7. Effect of point mutations in the specificity of the HP1 chromo domain for methyl-K9 H3 peptide. (A) Fluorescence polarization assays are illustrated for wild-type (circles), V26M (squares) and Y24F/A25P (triangles); measurements were performed at 25°C in 50 mM sodium phosphate pH 6 and 25 mM NaCl buffer. (B) The NMR chemical shift perturbations in backbone 1HN and 15N nuclei of the V26M mutant are marked on the structure (Ball et al., 1997) using the same view as in Figure 6C. Regions in magenta correspond to the sites where NMR signals disappear (residues 25–28, 43, 55, 62, 66 and 70) and regions in yellow (residues 54, 56 and 68) correspond to the sites with weighted chemical shift perturbations in the range 0.1 < Δδ < 0.2. Regions shown in white do not show any significant perturbation upon mutation.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.